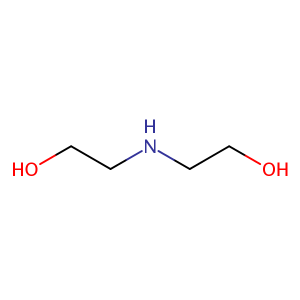
| CAS Number | 111-42-2 |
|---|---|
| Molecular Formula | C4H11NO2 |
| Molecular Weight | 105.137 |
| InChI Key | ZBCBWPMODOFKDW-UHFFFAOYSA-N |
| LogP | -1.44 |
| Synonyms |
|
Applications:
HPLC Separation of Glyphosate Reaction Intermediates and Impurities
March 3, 2010

Glyphosate and intermediates/impurities of production are separated on an Obelisc N HILIC/ion-exchange column by a combination of HILIC and ion-exchange mechanism. Method can be used in analysis of glyphosate, iminodiacetic acid, (N-phosphonomethyl)-imminodiacetic acid, diethanolamine and related impurities in reaction mixtures, waste and ground waters. Detection techniques are LC/MS, ELSD, CAD and UV.
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Ammonium Formate |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Herbicide, Hydrophilic, Ionizable |
| Analyzing Compounds | Glyphosate, IDA, PMIDA, DEA |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsGlyphosate
IDA (Iminodiacetic acid)
PMIDA (Phosphonomethyliminodiacetic acid)

HPLC Separation of Glyphosate Production Intermediates
January 13, 2010
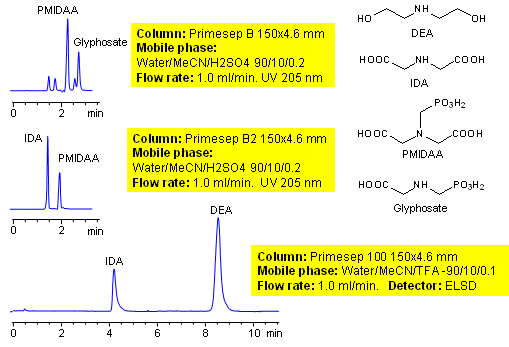
Glyphosate is a broad spectrum herbicide used to kill weeds. It is the most used herbicide. Glyphosate is an aminophosphonic analogue of the natural amino acid glycine. Glyphosate and its intermediates are very polar ionic compounds derived from glycine. Neither of intermediates can be retained on traditional reversed-phase columns. Two methods for glyphosate intermediates were developed on Primesep B, Primesep B2 and Primesep 100 columns.
| Column | Primesep B, Primesep B2, Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA, H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Insecticide, Herbicide, Fungicide, Hydrophobic, Ionizable |
| Analyzing Compounds | Glyphosate, PMIDAA, IDA |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDiethanolamine
Glyphosate
IDA (Iminodiacetic acid)
Iminodiacetic Acid
PMIDA (Phosphonomethyliminodiacetic acid)
Phosphonomethyliminodiacetic Acid
UV Detection