| CAS Number | 150-13-0 |
|---|---|
| Molecular Formula | C7H7NO2 |
| Molecular Weight | 137.139 |
| InChI Key | ALYNCZNDIQEVRV-UHFFFAOYSA-N |
| LogP | 0.83 |
| Synonyms |
|
Applications:
UV-Vis Spectrum of 4-Aminobenzoic Acid
July 11, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze 4-Aminobenzoic Acid check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

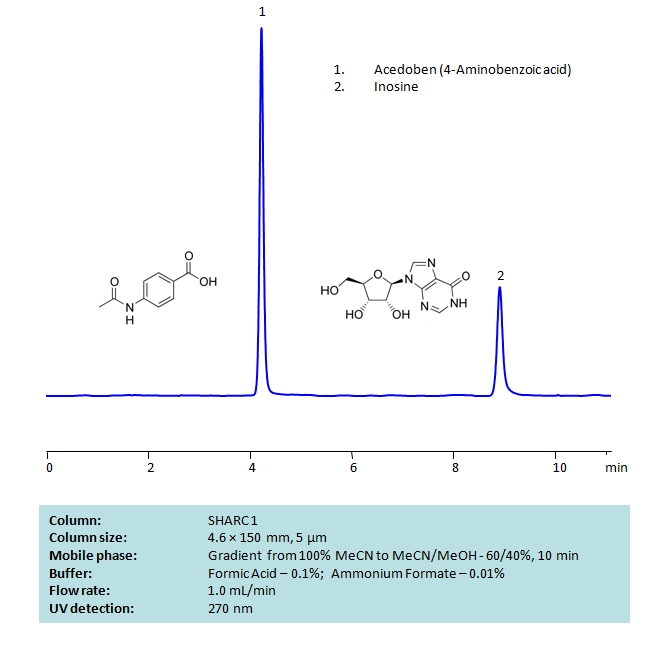
HPLC Separation of Acedoben and Inosine on SHARC 1 Column
December 21, 2020
HPLC Method for 4-Aminobenzoic Acid, Inosine on SHARC 1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of 4-Aminobenzoic Acid, Inosine.
Inosine pranobex is an antiviral drug. A combination of inosine and dimepranol acedoben (a salt of acetamidobenzoic acid and dimethylaminoisopropanol) has no effect on viral particles itself. Instead, it acts as an immunostimulant. It is most commonly used to treat the rare measles complication subacute sclerosing panencephalitis in conjunction with intrathecal interferon therapy.
Chromatography of these two compounds can be difficult due to their high polarity. But both compounds can be well retained and separated using anhydrous (water-free) conditions using HPLC on SHARC 1 column, which uses hydrogen-bonding as a separation mechanism. The method uses a gradient of acetonitrile (ACN) and methanol (MeOH) mobile phase with volatile buffer containing Formic Acid 0.1% and AmFm – 0.01%, making the method MS-compatible. Both compounds can also be UV detected at 270 nm.
| Column | SHARC 1, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/MeOH |
| Buffer | Formic Acid 0.1% , AmFm – 0.01% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm |
| Class of Compounds |
Nucleoside monophosphate, Hydrophilic, Ionizable |
| Analyzing Compounds | 4-Aminobenzoic Acid, Inosine |
Application Column
SHARC 1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Inosine

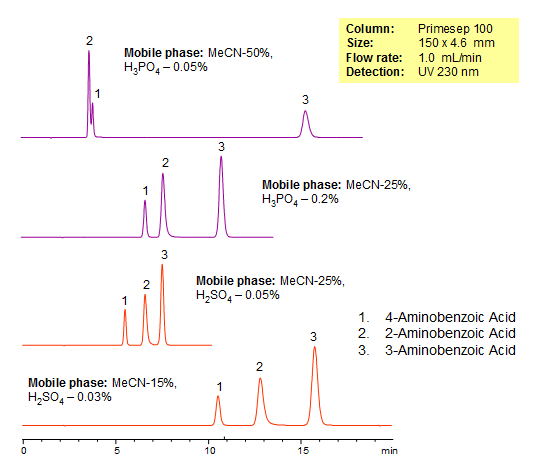
Separation of Isomers of Aminobenzoic Acid
October 15, 2015
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | H3PO4, H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Acid, Hydrophobic, Ionizable |
| Analyzing Compounds | 4-Aminobenzoic acid, 3-Aminobenzoic acid, 2-Aminobenzoic acid |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options3-Aminobenzoic Acid
4-Aminobenzoic Acid

USP Methods Analysis of Procainamide on Legacy L1 Column
June 21, 2012
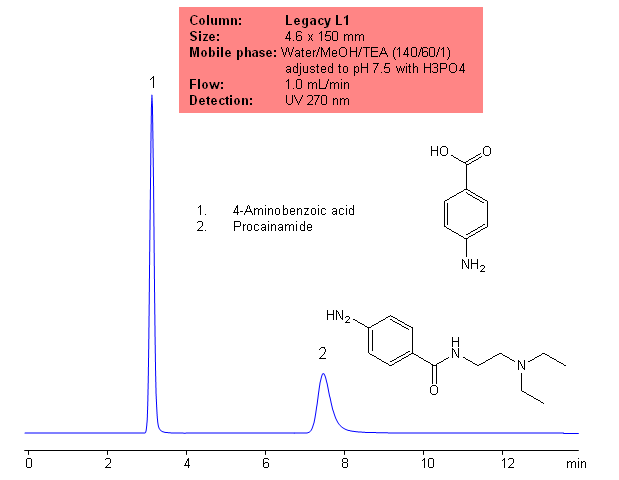
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Procainamide and 4-aminobenzoic acid
Mobile phase: WAter/MeOH/MeCN (70/70/90) with 7 mM sodium lauryl sulfate and 11mM phosphoric acid
Detection technique: UV
Reference: USP35: NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Water/MeOH/TEA (140/60/1) adjusted to pH 7.5 with H3PO4 |
| Buffer | TEAPh |
| Flow Rate | 1.5 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Drug, Nonsteroidal anti-inflammatory drug, Hydrophobic, Ionizable |
| Analyzing Compounds | Ibuprofen, benzophenone |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsProcainamide
Procainamide Hydrochloride

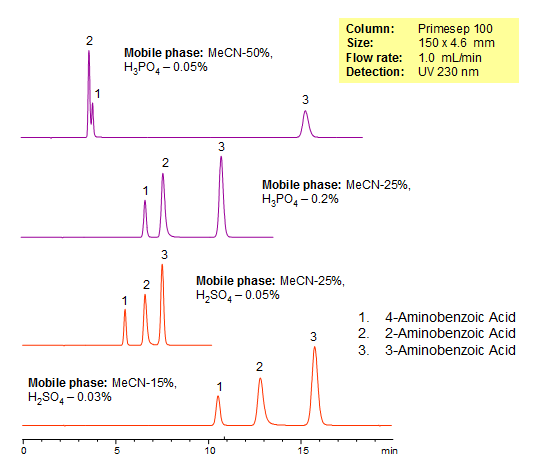
HPLC Separation of Isomers of Aminobenzoic Acid
March 27, 2011
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | H3PO4, H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Acid, Hydrophobic, Ionizable |
| Analyzing Compounds | 4-Aminobenzoic acid, 3-Aminobenzoic acid, 2-Aminobenzoic acid |
Aminobenzoic Acid is an organic acid that can be found in folic acid vitamins, grains, eggs, milk, and meat. Because it absorbs the UV light, it is used in sunscreen with SPF 15 or greater. The acid can cause damage to DNA and skin irritations in rare cases. Primesep 100, a reverse phase column, contains embedded acidic ionizable groups and can retain aminobenzoic acid. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
Isomers of organic compounds usually have very similar properties. Separation of such isomers by HPLC requires intensive screening of various columns and mobile phase conditions. In case of hydrophilic compounds, this task becomes even more challenging due to the lack of or limited retention on traditional reversed-phase (C18 or C8) HPLC columns. Three isomers of aminobenzoic acid (2-aminobenzoic acid, 3-aminobenzoic acid and 4-aminobenzoic acid) were successfully separated on a Primesep 100 reversed-phase/cation-exchange column. Baseline separation within 10 minutes was achieved and produced a perfect peak shapes for all three analytes. The method can be used a generic approach for separation of ionic hydrophobic and ionic basic isomers by mixed-mode chromatography.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options3-Aminobenzoic Acid
4-Aminobenzoic Acid