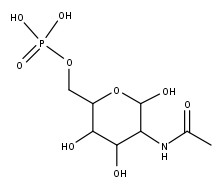
| CAS Number | 18191-20-3 |
|---|---|
| Molecular Formula | C8H16NO9P |
| Molecular Weight | 301.19 |
| InChI Key | BRGMHAYQAZFZDJ-RTRLPJTCSA-N |
| LogP | -4.2 |
| Synonyms |
|
Applications:
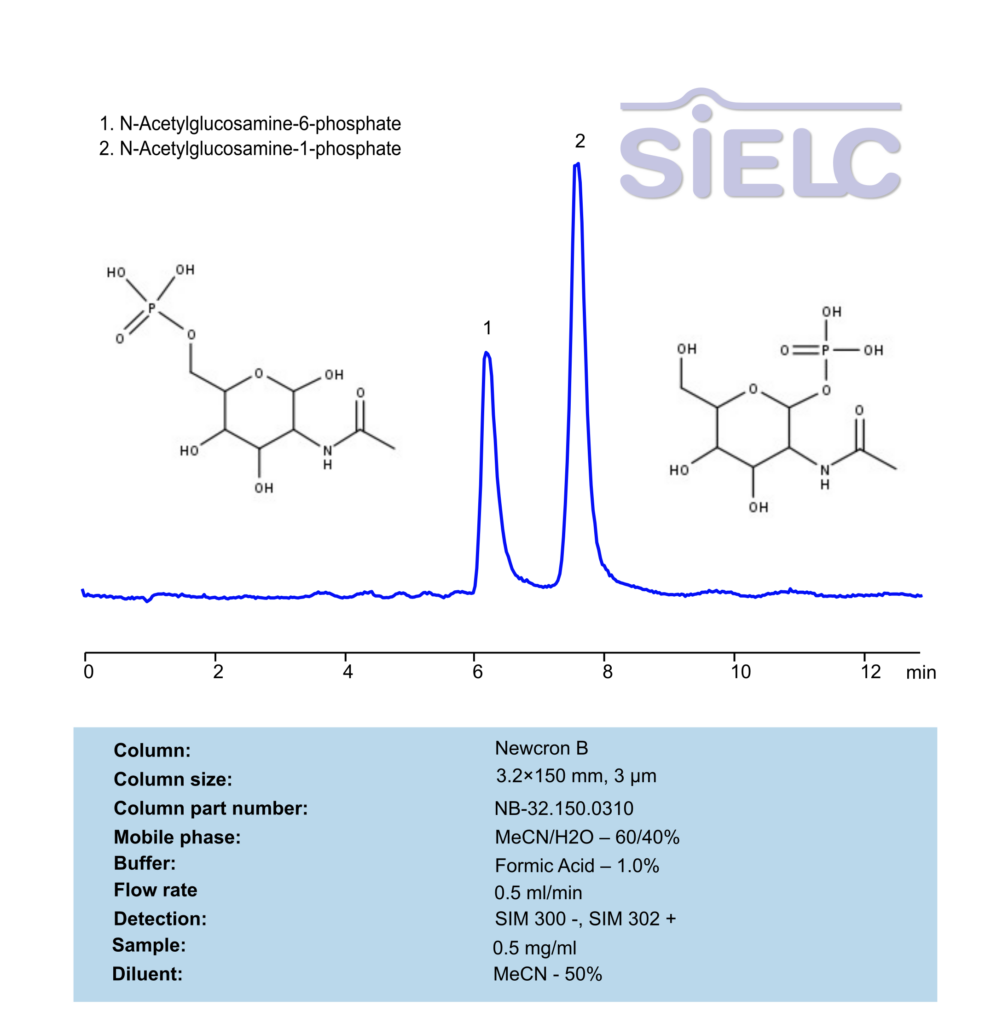
LCMS Method for Analysis of N-Acetylglucosamine-6-phosphate and N-Acetylglucosamine-1-phosphate on Newcrom B Column
December 10, 2024
HPLC Method for Analysis of N-Acetylglucosamine-1-phosphate, N-Acetylglucosamine-6-phosphate on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of N-Acetylglucosamine-1-phosphate, N-Acetylglucosamine-6-phosphate
N-Acetylglucosamine-6-phosphate (GlcNAc-6-P) and N-Acetylglucosamine-1-phosphate (GlcNAc-1-P) are two phosphorylated derivatives of N-acetylglucosamine (GlcNAc), a monosaccharide that is part of various biological structures, such as glycoproteins and glycosaminoglycans.
- N-Acetylglucosamine-6-phosphate (GlcNAc-6-P):
- This molecule is an intermediate in the biosynthesis of glycosaminoglycans like hyaluronic acid and chondroitin sulfate.
- It is involved in several metabolic pathways, including those related to the formation of UDP-GlcNAc, a precursor for glycosylation reactions.
- The 6-phosphate group is added to the carbon-6 position of the GlcNAc molecule.
- N-Acetylglucosamine-1-phosphate (GlcNAc-1-P):
- This compound is an important intermediate in the synthesis of glycoproteins and glycosaminoglycans.
- It plays a role in the biosynthesis of UDP-GlcNAc, which is used in glycosylation processes.
- The 1-phosphate group is attached to the carbon-1 position of the GlcNAc molecule.
- This compound can also participate in the formation of glycosaminoglycans like heparan sulfate and chondroitin sulfate.
Both GlcNAc-6-P and GlcNAc-1-P are involved in the regulation of metabolism and glycosylation, but they differ in the position of the phosphate group on the glucose structure.
N-Acetylglucosamine-6-phosphate (GlcNAc-6-P) and N-Acetylglucosamine-1-phosphate (GlcNAc-1-P) can be retained, separated and analyzed using an Newcrom B mixed-mode stationary phase column. The analysis employs a gradient method with a simple mobile phase consisting of water, acetonitrile (MeCN), and formic acid as a buffer. Detection is achieved using LC MS.
| Column | Newcrom B, 3.2 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 60/40 % |
| Buffer | Formic Acid – 1.0% |
| Flow Rate | 0.5 ml/min |
| Detection | SIM 300 -, SIM 302 + |
| Sample | 0.5 mg/ml |
| Injection volume | 1 µl |
| LOD* | 300 ppb |
| Class of Compounds | Amino sugars |
| Analyzing Compounds | N-Acetylglucosamine-1-phosphate, N-Acetylglucosamine-6-phosphate |
Application Column
Newcrom B
Column Diameter: 3.2 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
N-Acetylglucosamine-6-phosphate


