| CAS Number | 121-91-5 |
|---|---|
| Molecular Formula | C8H6O4 |
| Molecular Weight | 166.13 |
| InChI Key | QQVIHTHCMHWDBS-UHFFFAOYSA-N |
| LogP | 1.7 |
| Synonyms |
|
Applications:
UV-Vis Spectrum of Isophthalic acid
August 27, 2024
If you are looking for optimized HPLC method to analyze Isophthalic acid check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Analysis of Isophthalic acid on Primesep B Column
August 27, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Isophthalic acid on Primesep B by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies

High Performance Liquid Chromatography (HPLC) Method for Analysis of Isophthalic acid
Isophthalic acid is an aromatic dicarboxylic acid with the chemical formula C₆H₄(CO₂H)₂. It is a colorless crystalline solid and is one of the three isomers of benzene dicarboxylic acid, the others being phthalic acid and terephthalic acid. In isophthalic acid, the two carboxyl groups (−COOH) are positioned on the 1st and 3rd carbons of the benzene ring, making it a meta isomer.
Uses:
- Polyester production: Isophthalic acid is mainly used in the production of resins like polyethylene terephthalate (PET), which are important in the manufacturing of plastic bottles and fibers.
- Coatings and paints: It is also used in the formulation of alkyd resins for coatings and paints.
- Polyamide resins and epoxy curing agents: Isophthalic acid is used to enhance the chemical resistance and durability of these materials.
Isophthalic acid can be retained, separated and analyzed using a Primesep B mixed-mode stationary phase column. The analysis employs a gradient method with a simple mobile phase comprising water, acetonitrile (MeCN), and phosphoric acid as a buffer. This method allows for detection using UV 210 nm.
You can find detailed UV spectra of Isophthalic acid and information about its various lambda maxima by visiting the following link.
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 30-70%, 10 min |
| Buffer | H3PO4 – 0.4% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Limit of Detection | 5 ppb |
| Class of Compounds | Acid |
| Analyzing Compounds | Isophthalic acid |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Separation of Phthalic Acids using Hydrogen Bonding
June 18, 2012
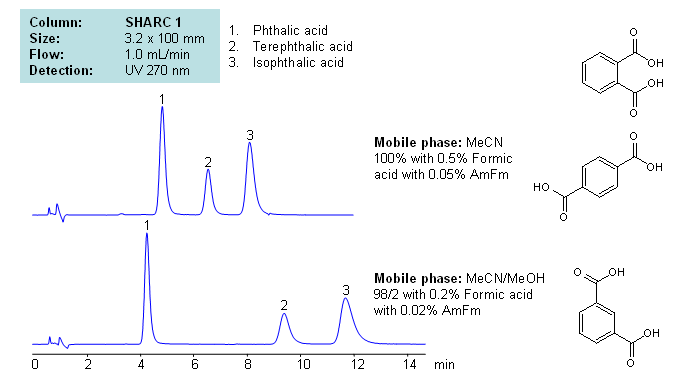
Phthalic acid, isophthalic acid and terephthalic acid are all isomers of each other. Being structurally similar, they can present difficulties to reverse-phase HPLC separation. Methods that require high organic concentrations in the mobile phase can cause dewetting in many reverse-phase columns. SHARC 1 column can be operated in anhydrous conditions and uses hydrogen bonding as the mechanism of separation. Here, phthalic acids were separated in pure acetonitrile (ACN), with the ability to adjust retention times by adding methanol (MeOH) to the mobile phase with formic acid and ammonium formate as buffer, making the method MS-compatible. Can also be UV detected at 270nm.
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Phthalic acid, Terephthalic acid, Isophthalic acid |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsPhthalic Acid
Terephthalic Acid



