| CAS Number | 87-08-1 |
|---|---|
| Molecular Formula | C16H18N2O5S |
| Molecular Weight | 350.391 |
| InChI Key | BPLBGHOLXOTWMN-MBNYWOFBSA-N |
| LogP | 2.09 |
| Synonyms |
|
Applications:
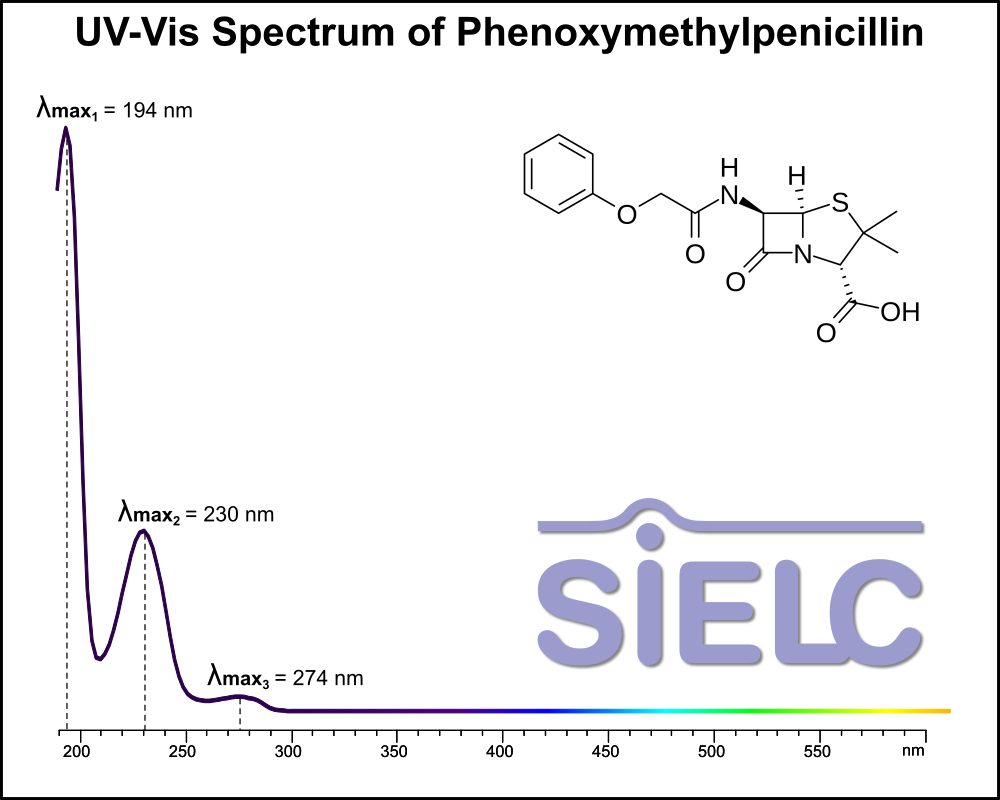
Uv-Vis Spectrum of Phenoxymethylpenicillin
February 16, 2026
If you are looking for optimized HPLC method to analyze Phenoxymethylpenicillin check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Separation of Phenoxymethylpenicillin
August 6, 2015
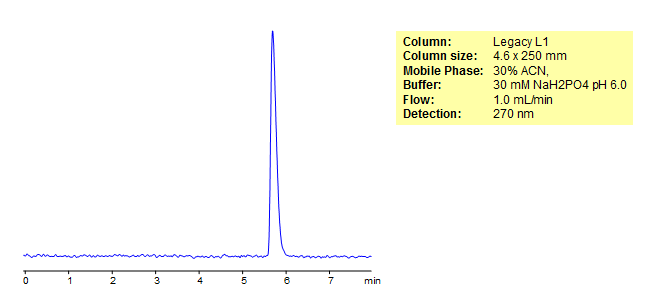
Phenoxymethylpenicillin (also known as penicillin V) is an antibiotic useful in the treatment of multiple bacterial infections such as those caused by Streptococcus pyogenes, Anthrax, Lyme Disease, Rheumatic fever, and blood infection prophylaxis in children with sickle cell diseases. Legacy L1 was used to retain Phenoxymethylpenicillin by reverse phase mechanism.Legacy L1 uses embedded C18 groups on porous silica and is useful for many USP HPLC applications. comparisons to Phenomenex columns are available by request.
| Column | Legacy L1, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 30% |
| Buffer | NaH2PO4 pH 6.0 – 30 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Hydrophobic, Ionizable |
| Analyzing Compounds | Phenoxymethylpenicillin |
&
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options