| CAS Number | 73-31-4 |
|---|---|
| Molecular Formula | C13H16N2O2 |
| Molecular Weight | 232.284 |
| InChI Key | DRLFMBDRBRZALE-UHFFFAOYSA-N |
| LogP | 1.20 |
| Synonyms |
|
Applications:
HPLC Separation of Amino Acids in Supplements Composition in Mixed-Mode
July 16, 2009
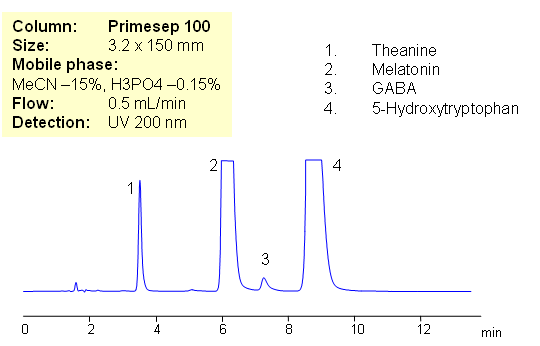
Amino acids are essential components of numerous formulation. Health supplements can contain various amino acids and vitamins and require quantitation of each ingredients. Amino acids are very polar compounds with limited or no retention in reversed-phase chromatography. The most common approaches are reversed-phase chromatography with ion-pairing reagent and hydrophilic interaction chromatography (HILIC). Underivatized amino acids can be retained by combination of reversed-phase and cation exchange mechanism on Primesep 100 mixed-mode. Retention time is controlled by amount of acetonitrile, buffer and buffer pH. Method does not require ion-pairing reagent. This method is for UV detection. LC/MS, ELSD or Corona CAD can be employed for analysis of amino acids with trifluoroacteic acid or ammonium formate in the mobile phase. This approach can be used for HPLC analysis of all underivatized amino acids.
| Column | Primesep 100, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 15/85% |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Theanine, Melatonin, GABA, 5- Hydroxytryptophan |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAmino Acids
Melatonin
Theanine
gamma-Aminobutyric Acid (GABA)