| CAS Number | 131707-25-0 |
|---|---|
| Molecular Formula | C22H25BrN2O3S |
| Molecular Weight | 447.4 |
| InChI Key | KCFYEAOKVJSACF-UHFFFAOYSA-N |
| LogP | 4.4 |
| Synonyms |
|
Applications:
Uv-Vis Spectrum of Arbidol
January 19, 2026

If you are looking for optimized HPLC method to analyze Arbidol (Umifenovir) check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

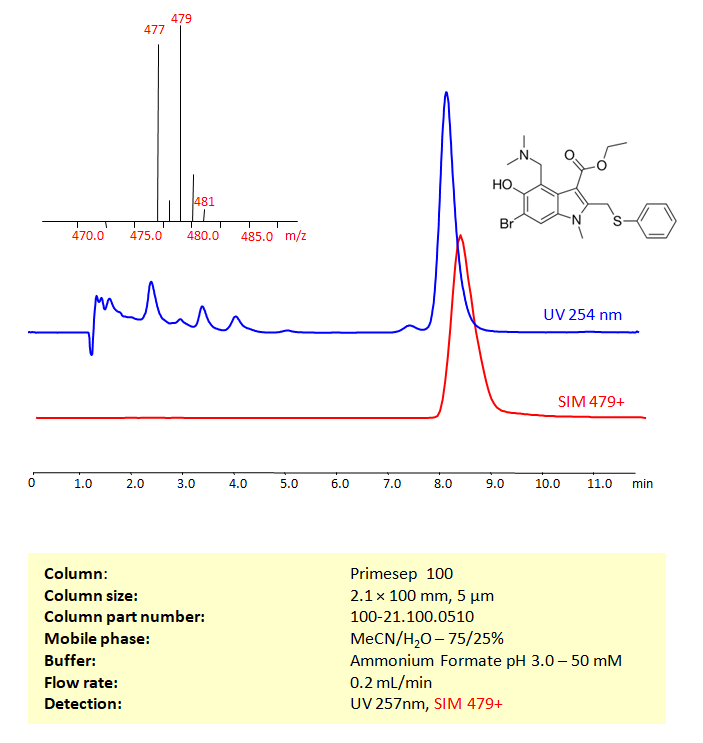
HPLC-MS Method for Analysis of Arbidol (Umifenovir) on Primesep 100 Column
August 22, 2023
High Performance Liquid Chromatography (HPLC) Method for Analysis of Arbidol (Umifenovir) on Primesep 100 by SIELC Technologies.
Separation type: Liquid Chromatography Mixed-mode
Umifenovir, commonly known by its trade name Arbidol, is an antiviral drug. Originally developed in Russia, it has been used primarily in Russia and China for the prophylaxis and treatment of influenza and other respiratory viral infections.
Mechanism of Action:
Umifenovir interferes with the viral ability to penetrate into host cells. It acts by inhibiting the fusion of the viral lipid membrane with the host cell membrane, thus preventing the virus from entering and infecting the cell.
Uses:
- Influenza: Umifenovir has been prescribed for influenza A and influenza B treatment.
- Other Viral Infections: Besides influenza, the drug has also been indicated for prophylaxis and treatment of acute respiratory viral infections (ARVI) and recurrent respiratory infections.
- Potential Role in COVID-19: In the wake of the COVID-19 pandemic, Umifenovir gained attention as a potential treatment for the virus. Some initial studies and clinical trials have been conducted to assess its efficacy against SARS-CoV-2. While it has been used in some countries as a part of COVID-19 treatment protocols, its efficacy specifically against COVID-19 is not firmly established, and research is ongoing.
Side Effects:
Though generally considered to be safe, some reported side effects of Umifenovir include digestive disturbances, allergic reactions, or elevated heart rate. However, side effects are generally rare. As always, any medication’s potential side effects should be discussed with a healthcare provider.
Availability:
While Umifenovir is available in countries like Russia and China, its acceptance and usage differ worldwide. For instance, it hasn’t been approved for antiviral use in the United States.
Arbidol can be retained, and analyzed on a Primesep 100 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a Ammonium formate as a buffer. This analysis method can be detected using UV at 254 nm, an Evaporative Light Scattering Detector (ELSD), or any other evaporative detection method (CAD, ESI-MS)
High Performance Liquid Chromatography (HPLC) Method for Analyses of Arbidol (Umifenovir)
Condition
| Column | Primesep 100, 2.1 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 75/25% |
| Buffer | Ammonium Formate pH 3.0- 50 mM |
| Flow Rate | 0.2 ml/min |
| Detection | UV 254 nm, LCMS SIM 479+ |
| Peak Retention Time | 8.17 min |
Description
| Class of Compounds | Drug |
| Analyzing Compounds | Arbidol (Umifenovir) |
Application Column
Primesep 100
Column Diameter: 2.1 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

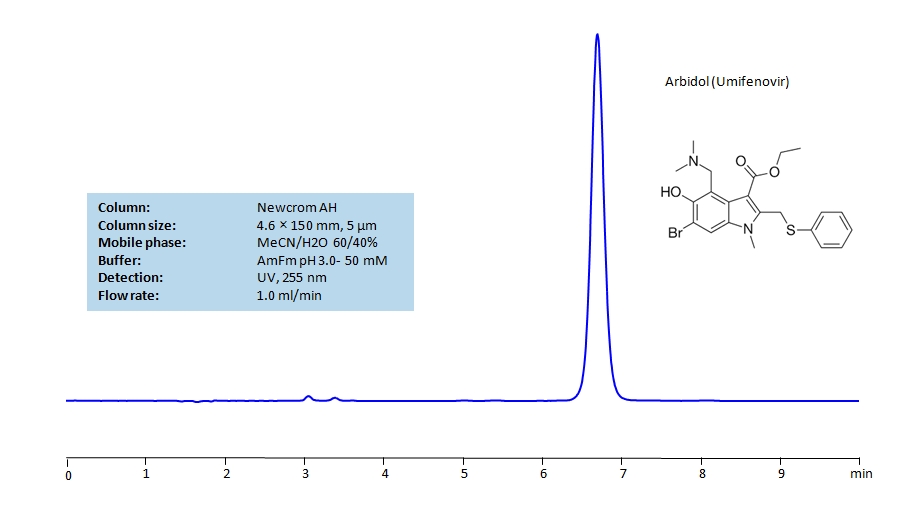
HPLC Determination of Arbidol (Umifenovir) on Newcrom AH Column
April 1, 2020
HPLC Method for Arbidol (Umifenovir) on Newcrom AH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Arbidol (Umifenovir).
Umifenovir is an antiviral medication with the chemical formula C22H25BrN2O3S. It is used to treat influenza in Russia and China for decades, but it is not approved for use in the United States by the Food and Drug Administration (FDA). It is considered a direct-acting antiviral as well as a host-targeting agent due to how it effects multiple stages of a viral lifecycle. Due to it’s wide-spread capabilities in covering both enveloped and non-enveloped RNA and DNA viruses, it is currently being investigated as treatment and prevention of COVID-19 caused by SARS-CoV-2 infections.
Arbidol (Umifenovir) can be retained and analyzed using the Newcrom AH stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN). Detection is performed using UV.
| Column | Newcrom AH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 60/40% |
| Buffer | AmFm pH 3.0- 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 255 nm, MS-compatible mobile phase |
| Class of Compounds | Hydrophobic, Drug |
| Analyzing Compounds | Arbidol (Umifenovir) |
Application Column
Newcrom AH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended