HPLC Method for Arbidol (Umifenovir) on Newcrom AH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Arbidol (Umifenovir).
Umifenovir is an antiviral medication with the chemical formula C22H25BrN2O3S. It is used to treat influenza in Russia and China for decades, but it is not approved for use in the United States by the Food and Drug Administration (FDA). It is considered a direct-acting antiviral as well as a host-targeting agent due to how it effects multiple stages of a viral lifecycle. Due to it’s wide-spread capabilities in covering both enveloped and non-enveloped RNA and DNA viruses, it is currently being investigated as treatment and prevention of COVID-19 caused by SARS-CoV-2 infections.
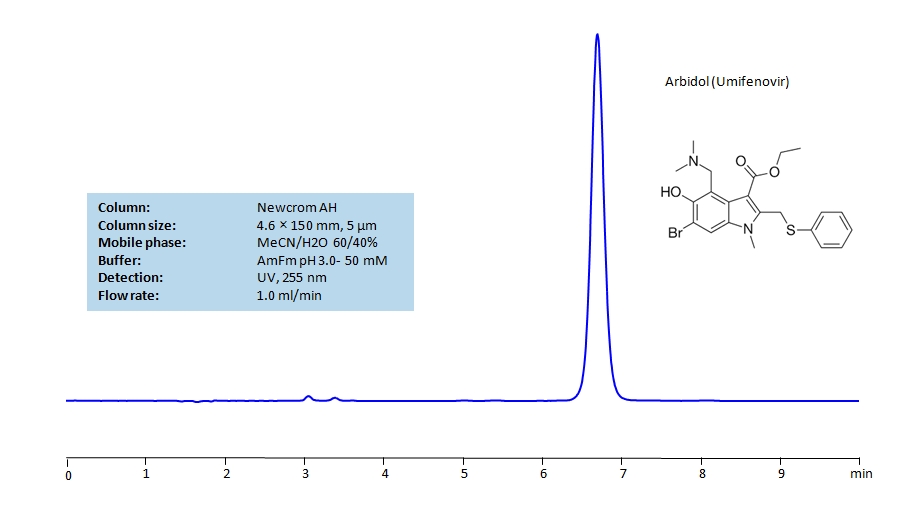
Arbidol (Umifenovir) can be retained and analyzed using the Newcrom AH stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN). Detection is performed using UV.
| Column | Newcrom AH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 60/40% |
| Buffer | AmFm pH 3.0- 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 255 nm, MS-compatible mobile phase |
| Class of Compounds | Hydrophobic, Drug |
| Analyzing Compounds | Arbidol (Umifenovir) |
Application Column
Newcrom AH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended