| CAS Number | 62-53-3 |
|---|---|
| Molecular Formula | C6H7N |
| Molecular Weight | 93.129 |
| InChI Key | PAYRUJLWNCNPSJ-UHFFFAOYSA-N |
| LogP | 0.9 |
| Synonyms |
|
Applications:
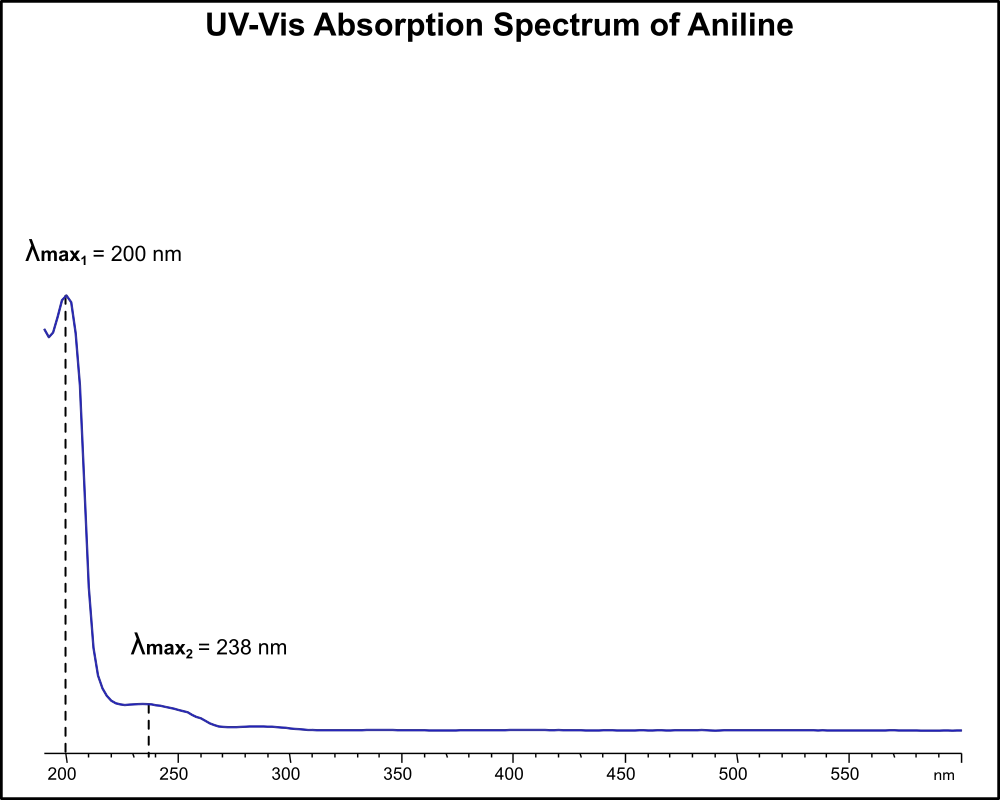
UV-Vis Absorption Spectrum of Aniline
December 12, 2025
If you are looking for optimized HPLC method to analyze Aniline check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Analysis of Aniline on Primesep 100 Column
August 1, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Aniline on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Aniline
Aniline is an organic compound consists of a phenyl group attached to an amino group.
Chemical Properties: Aniline is a primary aromatic amine. It is a colorless to slightly yellow liquid with a characteristic odor.
Production: Aniline is produced by the reduction of nitrobenzene or by the catalytic hydrogenation of nitrobenzene.
Uses: Aniline is a precursor to many industrial chemicals. It is used in the manufacture of polyurethane foam, rubber chemicals, dyes, agricultural chemicals, and pharmaceuticals. Aniline derivatives are also used as intermediates in the production of pigments and perfumes.
Toxicity: Aniline is toxic and can be absorbed through the skin, inhaled, or ingested. It can cause methemoglobinemia, a condition where hemoglobin is converted to methemoglobin, which cannot carry oxygen efficiently.
Aniline can be retained and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV 200 nm
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 45% |
| Buffer | H2SO4 -0.05% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Samples | 1.2 mg/ml in MeCN/H2O – 50/50% |
| Injection volume | 1 µl |
| LOD* | 10 ppb (200 nm) |
| Class of Compounds | Aromatic amines |
| Analyzing Compounds | Aniline |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

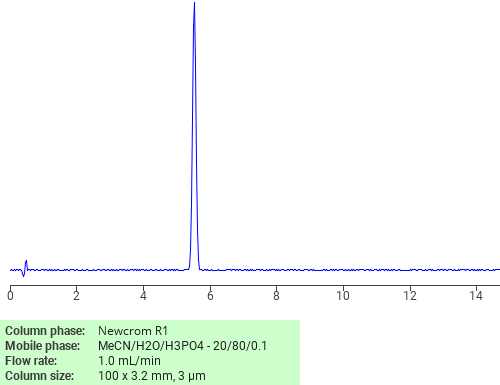
Separation of Aniline on Newcrom R1 HPLC column
May 16, 2018
Aniline is a compound made up of a benzene ring attached to an amino group. It is a precursor to many chemical compounds and used in rubber processing agents, dyes, and herbicides. Aniline can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particle columns are available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation of impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
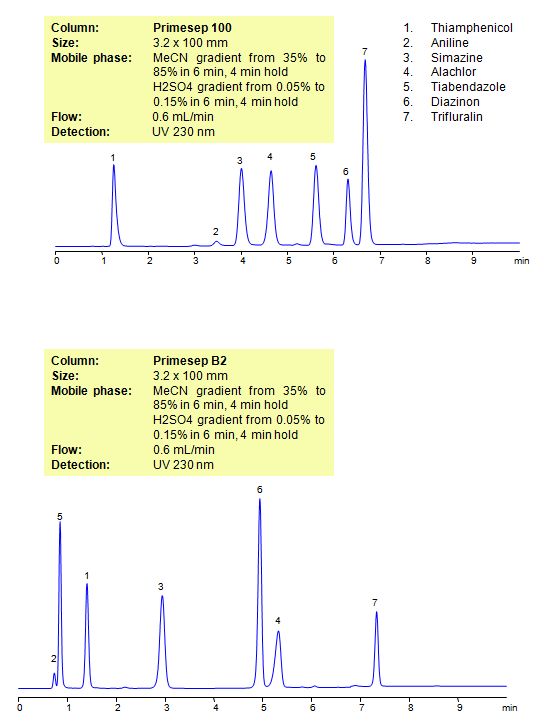
Select optionsHPLC Method for Analysis of Pesticides: Thiamphenicol, Aniline, Simazine, Alachlor, Thiabendazole, Diazinon, Trifluralin on Primesep B2 Column
July 11, 2017
| Column | Primesep B2, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 35-85%, 6 min , 4 min hold |
| Buffer | Gradient H2SO4 – 0.05- 0.15%, 6 min, 4 min hold |
| Flow Rate | 0.6 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Thiamphenicol, Aniline, Simazine, Alachlor, Thiabendazole, Diazinon, Trifluralin |
Application Column
Primesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAniline
Diazinon
Simazine
Thiabendazole
Thiamphenicol
Trifluralin