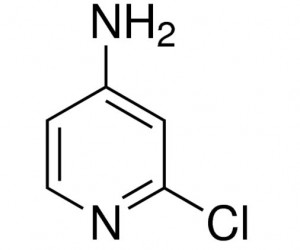
| CAS Number | 14432-12-3 |
|---|---|
| Molecular Formula | C5H5ClN2 |
| Molecular Weight | 128.559 |
| InChI Key | BLBDTBCGPHPIJK-UHFFFAOYSA-N |
| LogP | 1.1 |
| Synonyms |
|
Applications:
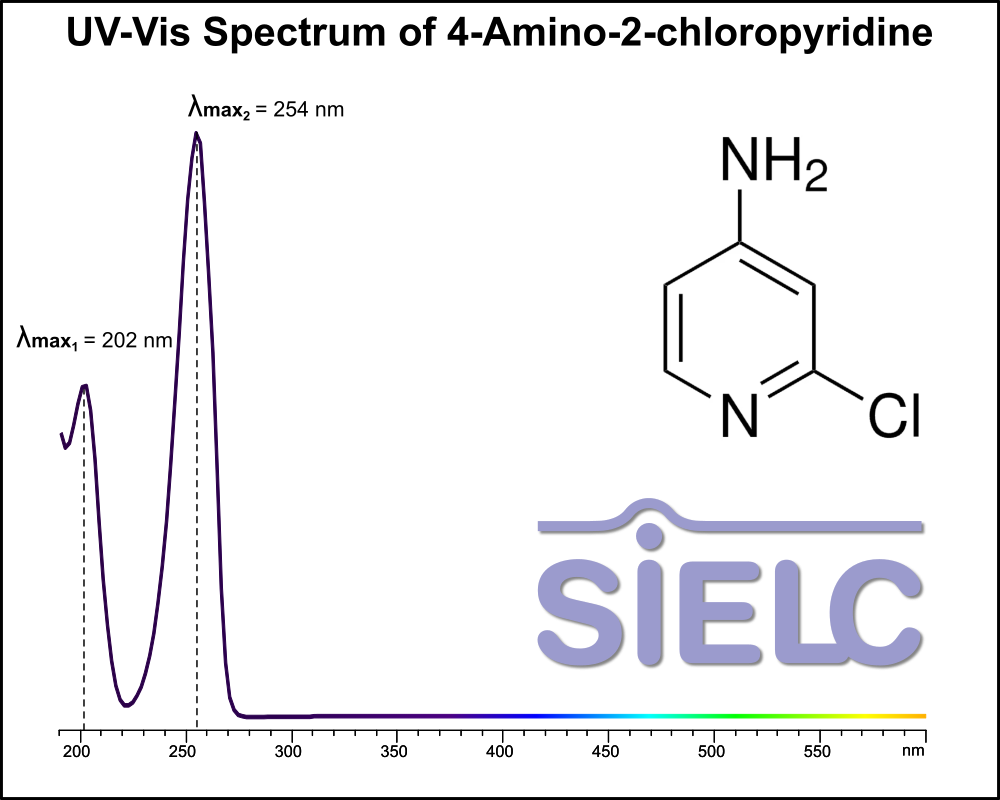
Uv-Vis Spectrum of 4-Amino-2-chloropyridine
February 25, 2026
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze 4-Amino-2-Chloropyridine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Analysis of 4-Amino-2-chloropyridine on Primesep 100 Column
July 30, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of 4-Amino-2-Chloropyridine on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of 4-Amino-2-Chloropyridine
4-Amino-2-chloropyridine is a chemical compound with the molecular formula C5H5ClN2. It’s a derivative of pyridine, featuring both an amino group (-NH2) and a chlorine atom as substituents.
4-Amino-2-chloropyridine is often used in organic synthesis and pharmaceuticals. It can serve as an intermediate in the production of various active pharmaceutical ingredients (APIs) and in the synthesis of other complex organic molecules.
4-Amino-2-Chloropyridine be retained and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV 200 nm
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 45% |
| Buffer | H2SO4 -0.05% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Samples | 1.0 mg/ml MeCN/H2O – 50/50% |
| Injection volume | 1 µl |
| LOD* | 8 ppb (200 nm) |
| Class of Compounds | Pyridines |
| Analyzing Compounds | 4-Amino-2-Chloropyridine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

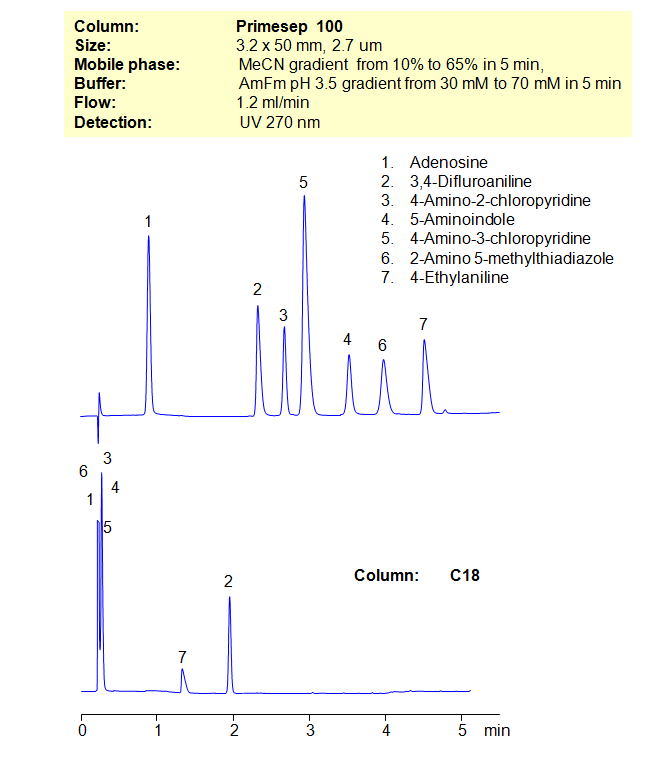
Separation of Model Compounds in Reversed-Phase and Mixed-Mode
April 25, 2019
HPLC Method for Adenosine, 3,4-Difluoroaniline, 4-Amino-2-Chloropyridine, 4-Amino-3-Chloropyridine, 2-Amino-5-Methylthiadiazole, 2-Amino-5-methyl-thiazole, 4-Ethylaniline, 5-Aminoindole on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Adenosine, 3,4-Difluoroaniline, 4-Amino-2-Chloropyridine, 4-Amino-3-Chloropyridine, 2-Amino-5-Methylthiadiazole, 2-Amino-5-methyl-thiazole, 4-Ethylaniline, 5-Aminoindole.
Many compounds are difficult, if not impossible, to separate on reverse-phase columns in HPLC. Other compounds cannot be separated on ion-exchange columns. That’s where the mixed-mode columns come in. By using a stationary phase with both hydrophobic and ion-exchange properties, allows the chromatographer to have additional controls over separation conditions. Here, we demonstrate the separation of compounds that can’t be achieved on a C18 column. By using both an organic gradient and buffer gradient of ammonium formate (AmFm), we can separate structurally similar compounds that can’t be separated on a reverse-phase column alone.
| Column | Solid-Core Primesep 100, 3.2 x 50 mm, 2.7 µm, 90 A, dual ended |
| Mobile Phase | Gradient MeCN – 10-60%, 5 min |
| Buffer | Gradient AmFm pH 3.5- 30 – 70 mM, 5 min |
| Flow Rate | 1.2 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Basic, Hydrophilic, Hydrophobic, Ionizable. |
| Analyzing Compounds | Adenosine, 3,4-Difluoroaniline, 4-Amino-2-Chloropyridine, 4-Amino-3-Chloropyridine, 2-Amino-5-Methylthiadiazole, 2-Amino-5-methyl-thiazole, 4-Ethylaniline, 5-Aminoindole |
Application Column
Solid-Core Primesep 100
Column Diameter: 3.2 mm
Column Length: 50 mm
Particle Size: 2.7 µm
Pore Size: 90 A
Attribute: none
Column options: dual ended
2-Amino-5-methyl-thiazole
3,4-Difluoroaniline
4-Amino-2-Chloropyridine
4-Amino-3-Chloropyridine
4-Ethylaniline
5-Aminoindole
Adenosine