| CAS Number | 99-56-9 |
|---|---|
| Molecular Formula | C6H7N3O2 |
| Molecular Weight | 153.14 |
| InChI Key | RAUWPNXIALNKQM-UHFFFAOYSA-N |
| LogP | 0.9 |
| Synonyms |
|
Applications:
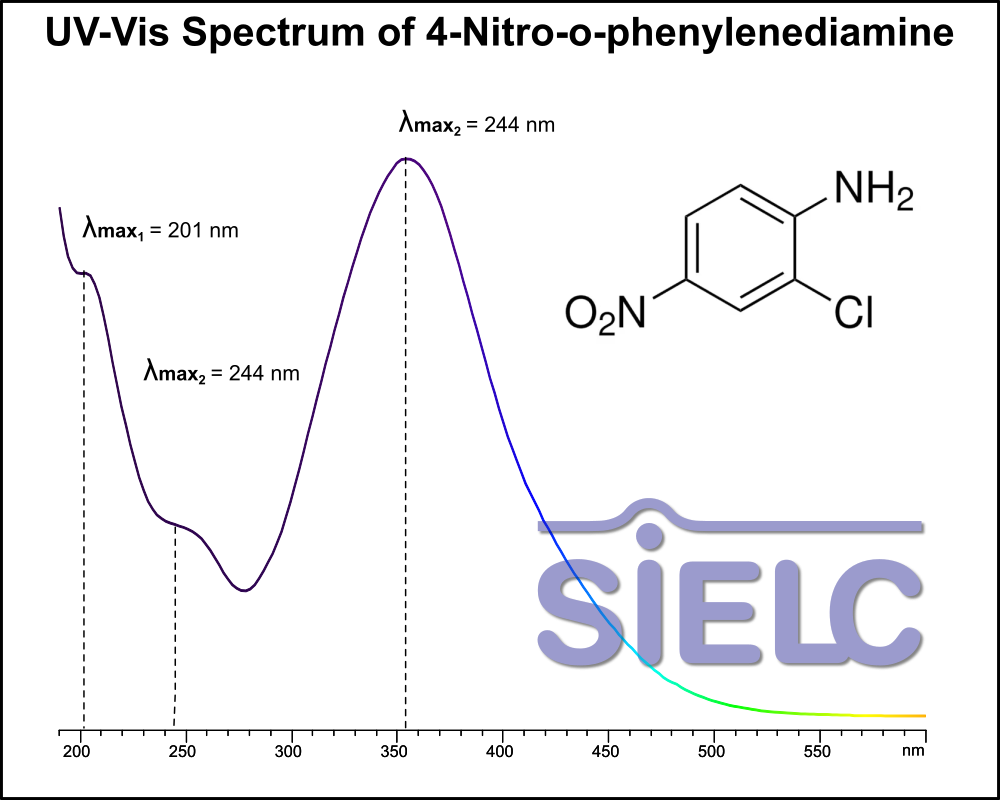
Uv-Vis Spectrum of 4-Nitro-o-phenylenediamine
February 12, 2026
If you are looking for optimized HPLC method to analyze 4-Nitro-o-phenylenediamine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

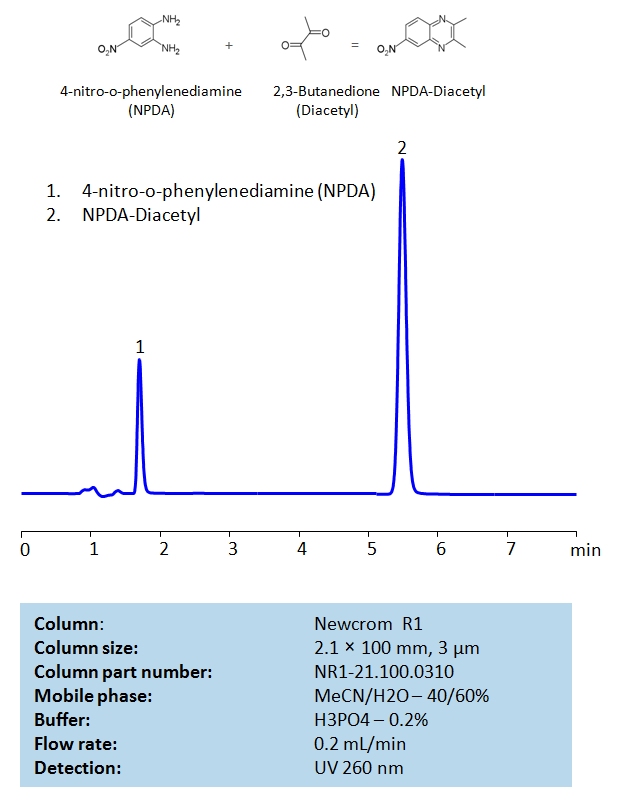
HPLC Determination of 2,3-Butanedione (Diacetyl) Using 4-Nitro-o-phenylenediamine as the Derivatization Reagent
July 8, 2021
HPLC Method for 2,3-Butanedione, 4-Nitro-o-phenylenediamine on Newcrom R1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Diacetyl.
Diacetyl is a natural by-product of fermentation and is known to be an important flavor compound in many food products. It is a reactive diketone in artificial butter flavors. The principal types of flavorings that use diacetyl are dairy flavors, particularly butter flavorings but also cheese, milk, and yogurt. Diacetyl is also sometimes an ingredient in the so-called brown flavors such as caramel, butterscotch, and coffee flavors.
The product of the derivatization of diacetyl with NPDA can be retained in HPLC on Newcrom R1 reverse-phase column with the simple isocratic mobile phase consisting of acetonitrile (MeCN), water and phosphoric acid (H3PO4). The analysis method can be UV detected at 260 nm.
Chemicals and Reagents
- The stock solutions of 0.1 mg/mL 2,3-Butanedione (Diacetyl) were prepared in distilled water.
- The stock solutions of 1.0 mg/mL 4-nitro-o-phenylenediamine (NPDA) were prepared in methanol.
Derivatization Procedure
- 0.10 mL of diacetyl stock solution,
- 0.10 mL of HCl (0.1 M),
- 0.60 mL of methanol, and
- 0.20 mL of NPDA stock solution were added to a test vial.
The total solution was sonicated for 20 minutes. The resulting solution was filtered through a 0.22 μm filter membrane and injected into the chromatographic system.
| Column | Newcrom R1, 2.1 x 100 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 40% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 0.2 ml/min |
| Detection | UV 260 nm |
| Class of Compounds | Ketone |
| Analyzing Compounds | 2,3-Butanedione, 4-Nitro-o-phenylenediamine |
Application Column
Newcrom R1
Column Diameter: 2.1 mm
Column Length: 100 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
4-Nitro-o-phenylenediamine