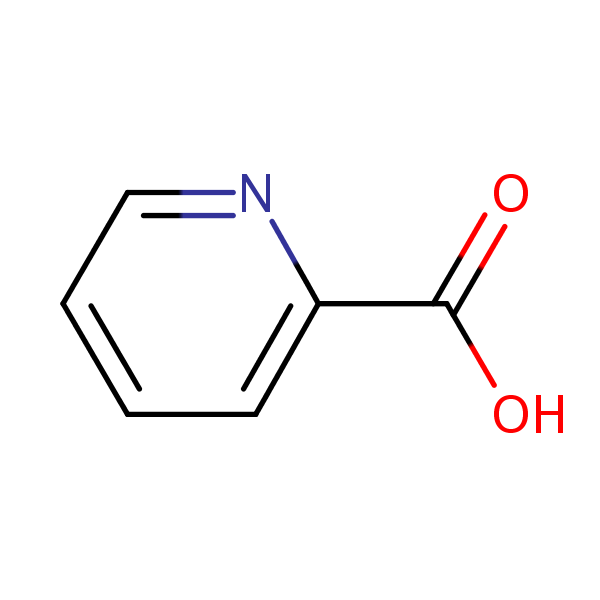
| CAS Number | 98-98-6 |
|---|---|
| Molecular Formula | C6H5NO2 |
| Molecular Weight | 123.112 |
| InChI Key | SIOXPEMLGUPBBT-UHFFFAOYSA-N |
| LogP | 0.72 |
| Synonyms |
|
Applications:
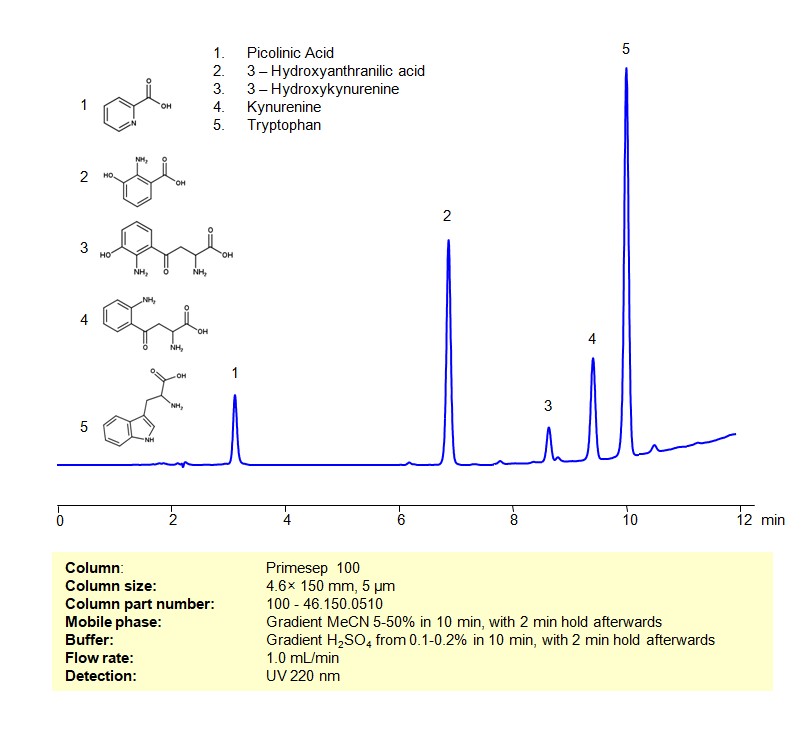
HPLC Method for Separation of a Mixture of Tryptophan and its Catabolites on Primesep 100 Column
October 3, 2023
High Performance Liquid Chromatography (HPLC) Method for Analysis of Mixture of Tryptophan and its Catabolites on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
Tryptophan and its catabolites participate in several biological pathways, having roles in protein synthesis, serving as precursors to bioactive molecules, and influencing several physiological processes. Here’s an overview considering a mixture of tryptophan and its catabolites:
Tryptophan:
- Essential Amino Acid: Tryptophan is a precursor to several important compounds, including serotonin and melatonin.
- In Protein Synthesis: Incorporated into proteins during protein synthesis.
Catabolites:
1. Serotonin:
- Neurotransmitter: Regulates mood, appetite, and sleep, among other functions.
- Derivative: Melatonin, which regulates the sleep-wake cycle.
2. Kynurenine Pathway (Major catabolic pathway of tryptophan):
- Kynurenine: An intermediate and precursor to several bioactive compounds.
- Kynurenic Acid: An NMDA receptor antagonist, believed to have neuroprotective effects.
- Xanthurenic Acid: Its physiological roles are still being explored, but it’s often studied for its relation to diabetes and neurological conditions.
- 3-Hydroxykynurenine: Can generate reactive oxygen species, potentially contributing to cellular stress.
- Quinolinic Acid: A neuroactive metabolite that can act as an NMDA receptor agonist.
3. Indoleamine 2,3-dioxygenase (IDO) Pathway:
- Tryptophan can be degraded into several catabolites via the IDO pathway, influencing immune response and cell proliferation.
.
Tryptophan and its Catabolites can be retained, separated and analyzed on a Primesep 100 mixed-mode stationary phase column using an gradient analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid as a buffer. This analysis method can be detected using UV at 220 nm.
High Performance Liquid Chromatography (HPLC) Method for Analyses of Mixture of Tryptophan and its Catabolites
Condition
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | Gradient MeCN 5-50% in 10 min, with 2 min hold afterwards |
| Buffer | Gradient H2SO4 from 0.1-0.2% in 10 min, with 2 min hold afterwards |
| Flow Rate | 1.0 ml/min |
| Detection | UV 220 nm |
Description
| Class of Compounds | Essential Amino Acid Tryptophan and its Catabolites |
| Analyzing Compounds | Tryptophan, Picolinic Acid, Kynurenine, 3-Hydroxykynurenine, 3-Hydroxyanthranilic acid |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
3-Hydroxykynurenine
Kynurenine
Picolinic Acid
Tryptophan

HPLC Separation of Pyridinecarboxylic Acids
March 27, 2011
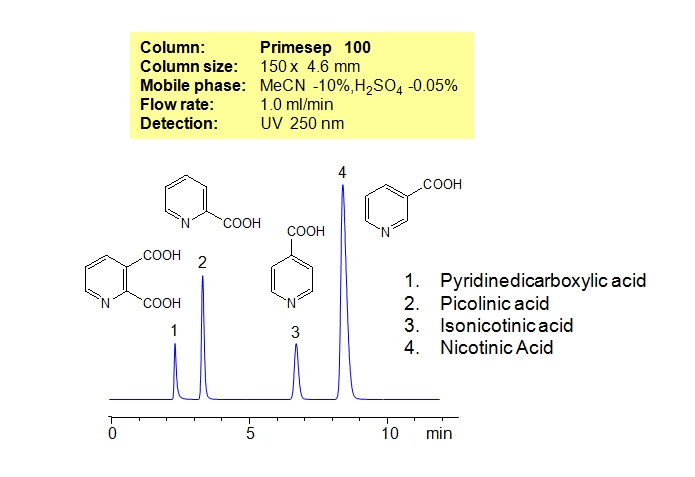
Pyridinecarboxylic acids exist as three isomers with different position of carboxylic acid relative to nitrogen in pyridine. Three isomers of pyridinecarboxylic acid (picolinic or 2-pyridinecarvoxylic acid, niacin or 3-pyridinecarboxylic acid, isonicotinic or 4-pyridinecarboxylic acid), along with pyridinedicarboxylic acid, are separated on a Primesep 100 column. Pyridinecarboxylic acids have a similar empirical formula, and are very similar in terms of hydrophobicity and ionic properties. Small differences in these properties are enough to achieve good separation on cation-exchange mixed-mode HPLC column like Primesep 100. Retention time for all compounds is controlled by the amount of acetonitrile and amount of ions in the mobile phase. Ions in the mobile phase can be created by organic and inorganic acids and corresponding salt buffers. Various detection techniques can be used for monitoring pyridinecarboxylic acids. Other ionizable isomers can be successfully separated on mixed-mode columns.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsNicotinic Acid/Niacin (3-pyridinecarboxylic acid)
Organic Acids
Picolinic Acid
Pyridinedicarboxylic Acid