| CAS Number | 108-48-5 |
|---|---|
| Molecular Formula | C7H9N |
| Molecular Weight | 107.156 |
| InChI Key | OISVCGZHLKNMSJ-UHFFFAOYSA-N |
| LogP | 1.7 |
| Synonyms |
|
Applications:
Generic Screening Method for Complex Mixtures on Primesep 200
October 15, 2015
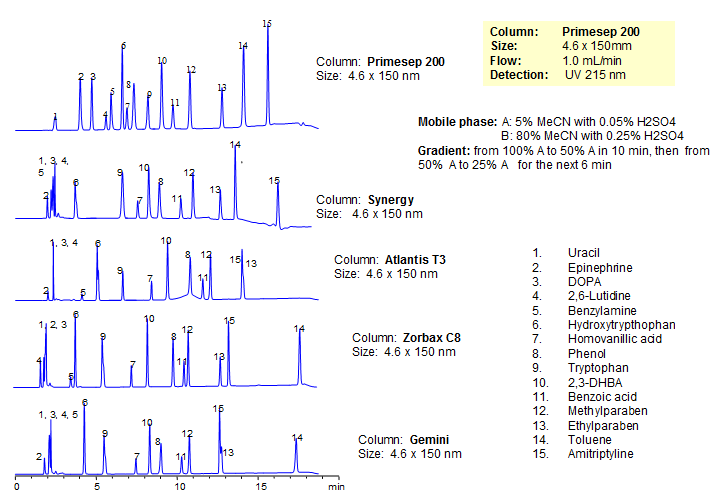
| Column | Primesep 200, 4.6*150 mm 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Epinephrine, DOPA, 2,6-Lutidine, Benzylamine, Hydroxytrypthophan, Homovanillic acid, Phenol, Tryptophan , 2,3-DHBA, Benzoic acid, Methylparaben, Ethylparaben, Toluene, Amitriptyline |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
2,6-Lutidine
Amitriptyline
Benzoic Acid
Benzylamine
DOPA (3,4-dihydroxy-L-phenylalanine)
Epinephrine
Ethylparaben
Homovanillic Acid
Hydroxytryptophan
Methylparaben
Phenol
Toluene
Tryptophan
Uracil

HPLC Separation of Amino Acids, Bases, Acids, and Neutrals on Obelisc R
March 3, 2007
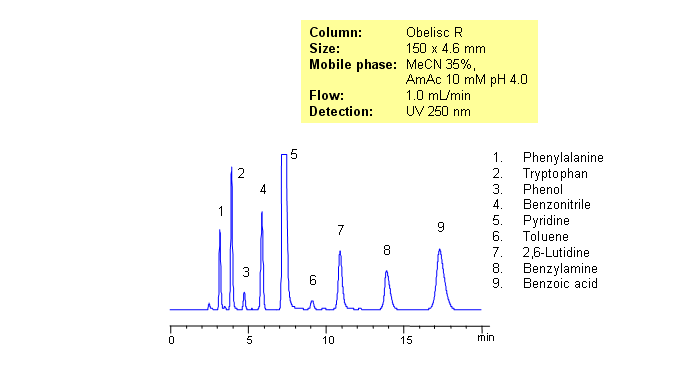
Separating basic, acidic and zwitterionic compounds in one run in reverse-phase HPLC can be very challenging. The methods might require the use of ion-pairing reagents and complex gradients that can make MS-compatibility difficult. Obelisc R column which has both positive and negative ion-pairs embedded in the stationary phase allows for fine tuning and separation of a wide range of compounds with different ionic properties. Acids, bases, amino acids and neutral compounds were separated isocratically in one run using a simple MS-compatible mobile phase of acetonitrile (ACN) and water with Ammonium Acetate (AmAc) buffer. Can also be UV detected at 250nm.
| Column | Obelisc R, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | AmAc 10 mM pH 4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Bases, Neutral, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Amino acids |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsBenzoic Acid
Benzonitrile
Benzylamine
Phenol
Phenylalanine
Pyridine
Toluene
Tryptophan