| CAS Number | 1628606-05-2 |
|---|---|
| Molecular Formula | C23H25N7O2 |
| Molecular Weight | 431.5 |
| InChI Key | TVGAHWWPABTBCX-UHFFFAOYSA-N |
| LogP | 1.5 |
| Synonyms |
|
Applications:
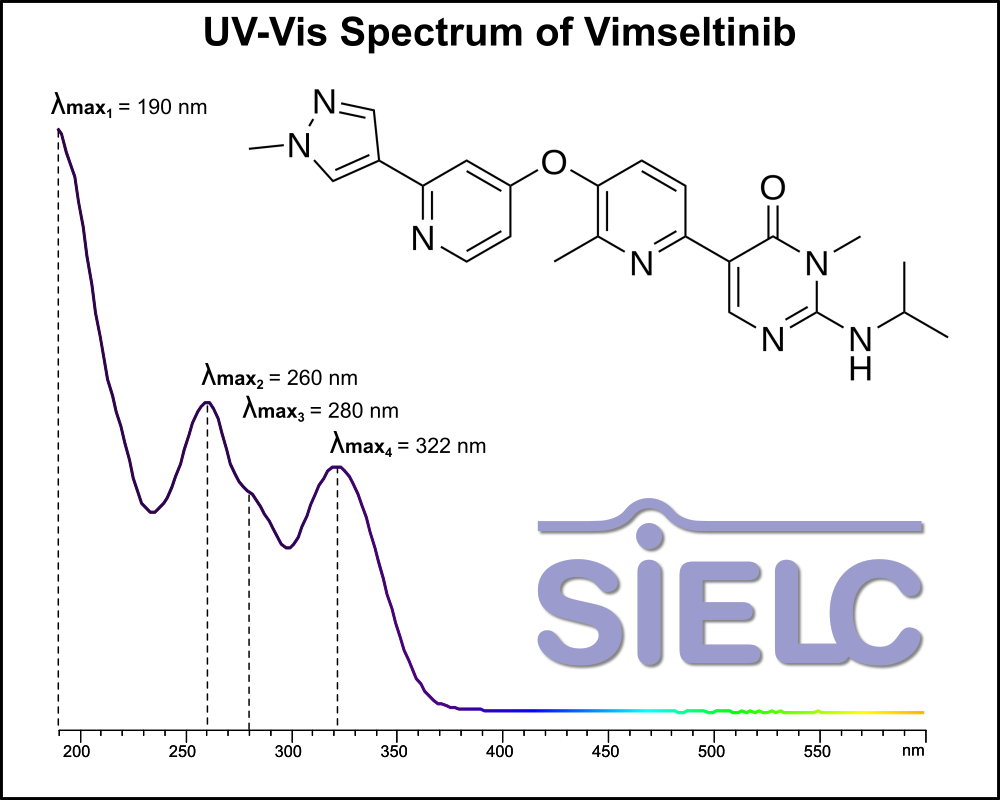
Uv-Vis Spectrum of Vimseltinib
February 18, 2026
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Vimseltinib check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

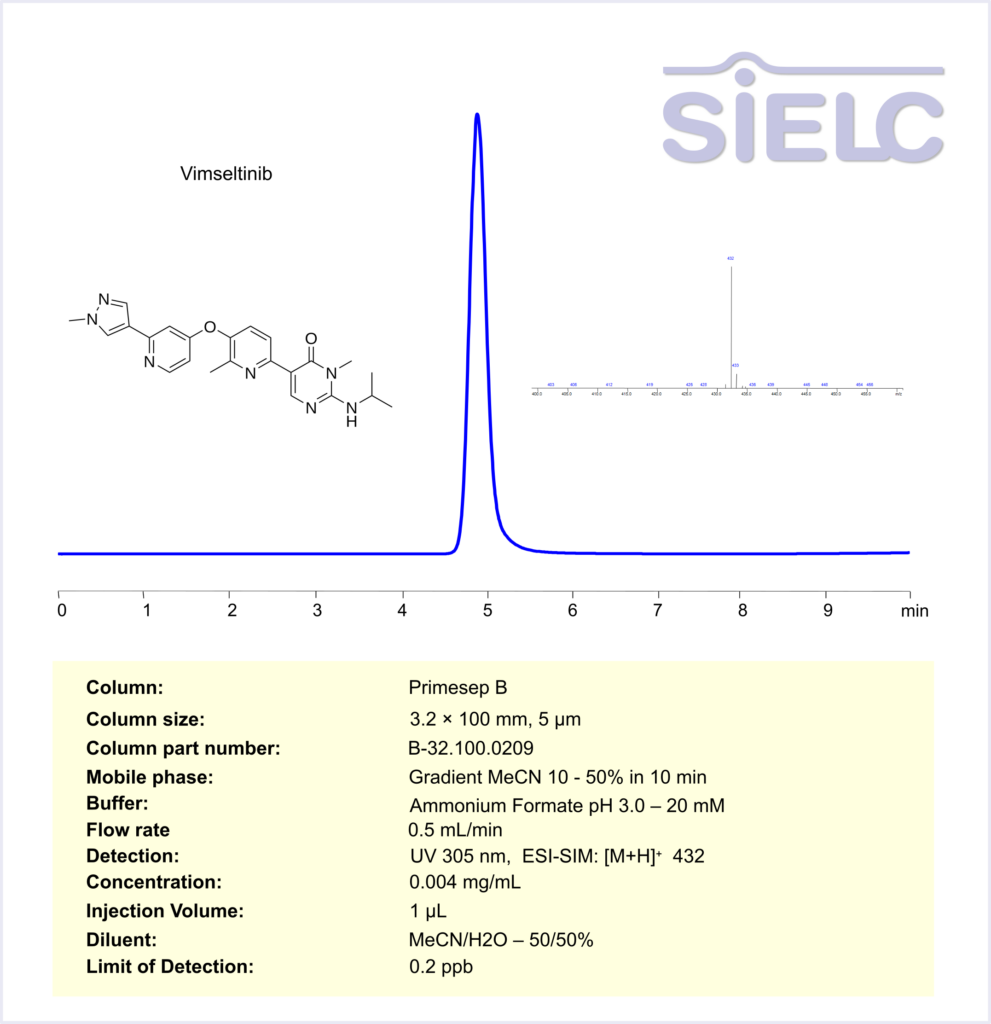
HPLC-MS Method for the Analysis of Vimseltinib on Primesep B Column
March 4, 2025
HPLC Method for Vimseltinib on Primesep B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Vimseltinib
Vimseltinib, sold under the brand name Romvimza, is an oral tyrosine kinase inhibitor designed to treat symptomatic tenosynovial giant cell tumor (TGCT), a rare and locally aggressive tumor that affects the joints. It specifically targets and inhibits the colony-stimulating factor 1 receptor (CSF1R), a key factor in the development and survival of macrophages, thus helping to reduce inflammation and tumor growth. In February 2025, the U.S. Food and Drug Administration (FDA) granted full approval for Vimseltinib following the successful results of the Phase 3 MOTION trial.
Vimseltinib can be retained and analyzed using the Primesep B stationary phase column. The analysis utilizes an gradientc method with a simple mobile phase consisting of water, acetonitrile (MeCN), and Ammonium formate. Detection is performed using UV at 305 nm. Mass spectrometric detection was carried out in ESI-SIM mode, monitoring the ions [M+H]⁺ at m/z 432
| Column | Primesep B, 3.2 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 10-50%, 10 mn |
| Buffer | Ammonium Formate pH 3.0 – 20 mM |
| Flow Rate | 0.5 ml/min |
| Detection | UV 305 nm, ESI-SIM: [M+H]⁺ 432 |
| LOD | 0.2 ppb |
*LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
| Class of Compounds | Drug |
| Analyzing Compounds | Vimseltinib |
Application Column
Primesep B
Column Diameter: 3.2 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
LC MS Detection