| CAS Number | 7491-74-9 |
|---|---|
| Molecular Formula | C6H10N2O2 |
| Molecular Weight | 142.159 |
| InChI Key | GMZVRMREEHBGGF-UHFFFAOYSA-N |
| LogP | -1.54 |
| Synonyms |
|
Applications:
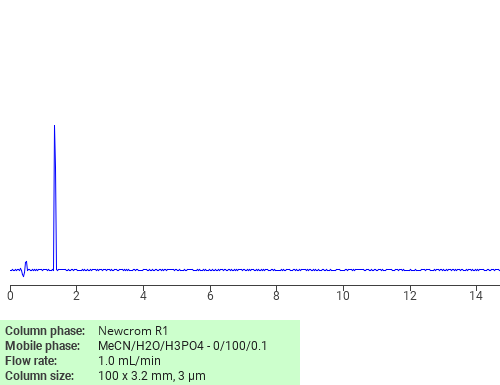
HPLC Method for Simultaneous Analysis of Piracetam and Levetiracetam in Pharmaceuticals
April 1, 2021
HPLC Method for Piracetam, Levetiracetam on Newcrom R1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Piracetam and Levetiracetam
Piracetam has been marketed as a treatment for myoclonus, vertigo, dyslexia, and sickle cell anemia. It is approved for use across many European countries, but is not approved by the FDA in the United States. It’s chemical formula is C6H10N2O2.
Levetiracetam, on the other hand, is an FDA Approved common treatment for generalized epilepsy and the prevention of seizures. It is currently unknown how exactly levetiracetam works as a treatment as it does not exhibit pharmacologic actions similar to other anticonvulsants. It’s chemical formula is C8H14N2O2
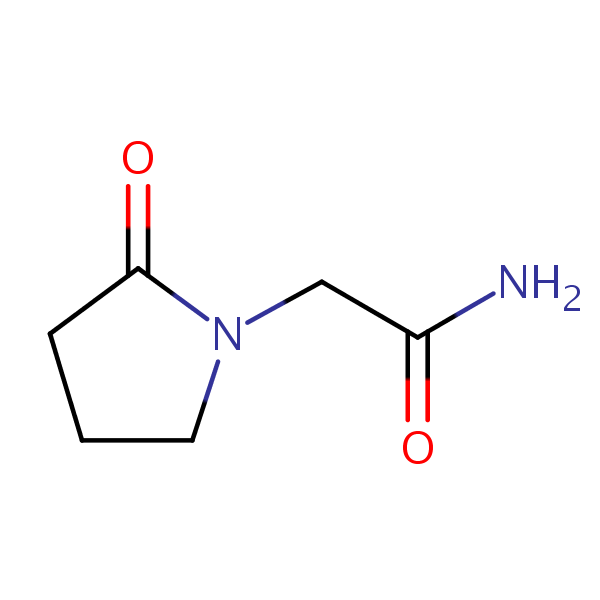
These racetams can be detected in the low UV regime. There are two effective methods for retaining, separating, and analyzing these compounds. The first uses a SHARC 1 hydrogen-bonding column and a mobile phase consisting of acetonitrile (MeCN) and water without a buffer. The second uses Newcrom R1 mixed-mode column and a mobile phase consisting of acetonirile (MeCN) and water with a sulfuric acid (H2SO4) buffer. Either analysis method can be UV detected at 200 nm with high resolution.
| Column | SHARC 1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O -98/2% |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Column | Newcrom R1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O -10/90% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Class of Compounds |
Drug, Racetam |
| Analyzing Compounds | Piracetam, Levetiracetam |
Application Column
SHARC 1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Newcrom R1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Piracetam

HPLC Determination of Piracetam on SHARC 1 Column
April 1, 2021
HPLC Method for Piracetam on SHARC 1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Piracetam
Piracetam has been marketed as a treatment for myoclonus, vertigo, dyslexia, and sickle cell anemia. It is approved for use across many European countries, but is not approved by the FDA in the United States. It’s chemical formula is C6H10N2O2.
[compound] can be detected in the low UV regime. Using a SHARC 1 hydrogen-bonding column and a mobile phase consisting of acetonitrile (MeCN) and water without a buffer, Piracetam can be retained, measured, and analyzed. This method can be UV detected at 200 nm with very high resolution
| Column | SHARC 1, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O -98/2% |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Class of Compounds |
Drug, Racetam |
| Analyzing Compounds | Piracetam |
Application Column
SHARC 1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

Separation of Piracetam on Newcrom R1 HPLC column
February 16, 2018
Piracetam can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options