| CAS Number | 51803-78-2 |
|---|---|
| Molecular Formula | C13H12N2O5S |
| Molecular Weight | 308.310 |
| InChI Key | HYWYRSMBCFDLJT-UHFFFAOYSA-N |
| LogP | 2.60 |
| Synonyms |
|
Applications:
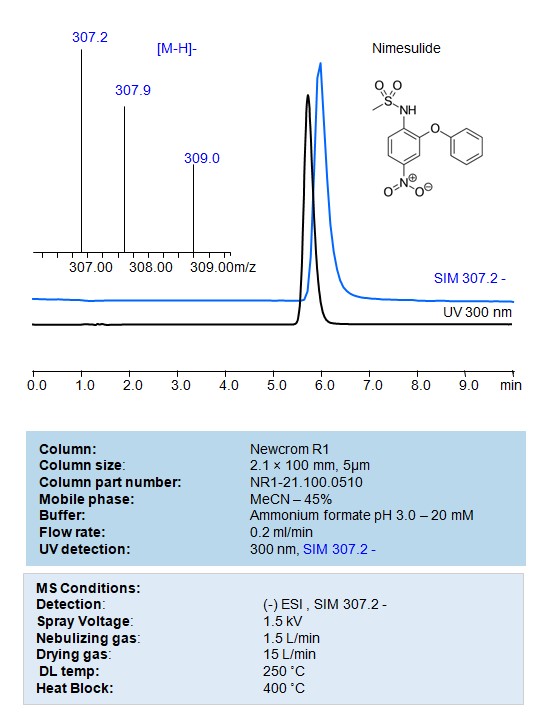
HPLC – MS Method for Analysis of Nimesulide on Newcrom R1 Column
October 4, 2023
HPLC Method for Analysis of Nimesulide on Newcrom R1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Nimesulide
Nimesulide is a non-steroidal anti-inflammatory drug (NSAID) that possesses analgesic and antipyretic properties. Below is some information about Nimesulide:
Mechanism of Action:
- COX Inhibition: Nimesulide inhibits the enzyme cyclooxygenase, specifically COX-2, which is responsible for the synthesis of prostaglandins, compounds that play a key role in promoting inflammation, pain, and fever.
Clinical Uses:
- Pain Management: Used to manage acute pain.
- Osteoarthritis: Utilized in the treatment of pain and inflammation associated with osteoarthritis.
- Primary Dysmenorrhea: Sometimes prescribed for managing pain during menstruation.
Safety Note:
- Due to concerns regarding liver toxicity, the use of Nimesulide is restricted or banned in some countries.
- It is important to use Nimesulide under medical supervision and strictly adhere to the prescribed dosage to minimize potential risks.
Synthesis:
- Nimesulide is synthesized via various chemical routes which generally involve the condensation of 4-nitro-2-phenoxyaniline with formaldehyde and subsequent sulfonation of the resulting methylene intermediate.
Nimesulide can be retained, separated and analyzed on a Newcrom R1 reverse phase stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a ammonium format as a buffer. This analysis method can be detected using UV at 300 nm, an Evaporative Light Scattering Detector (ELSD), or any other evaporative detection method (CAD, ESI-MS)
| Column | Newcrom R1, 2.1 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 50%, |
| Buffer | Ammonium Formate pH 3.0-20 mM |
| Flow Rate | 0.2 ml/min |
| Detection | UV 300 nm, SIM 307.2- |
| Spray Voltage: | 1.5 kV |
| Nebulizing gas: | 1.5 L/min |
| Drying gas: | 15 L/min |
| DL temp: | 250 ˚C |
| Heat Block: | 400 ˚C |
| Class of Compounds | Drug |
| Analyzing Compounds | Nimesulide |
Application Column
Newcrom R1
Column Diameter: 2.1 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

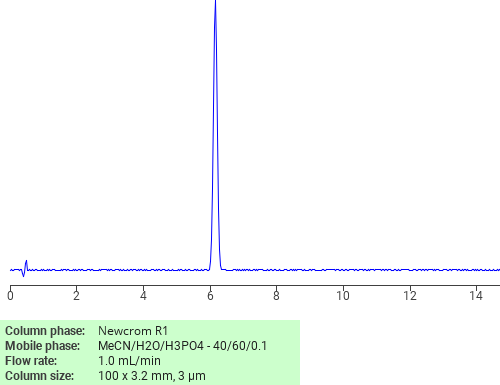
Separation of Nimesulide on Newcrom R1 HPLC column
February 16, 2018
Nimesulide can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options