HPLC Method for DMF (Dimethylformamide), DMSO (Dimethyl sulfoxide) on SHARC 1 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of DMF (Dimethylformamide), DMSO (Dimethyl sulfoxide).
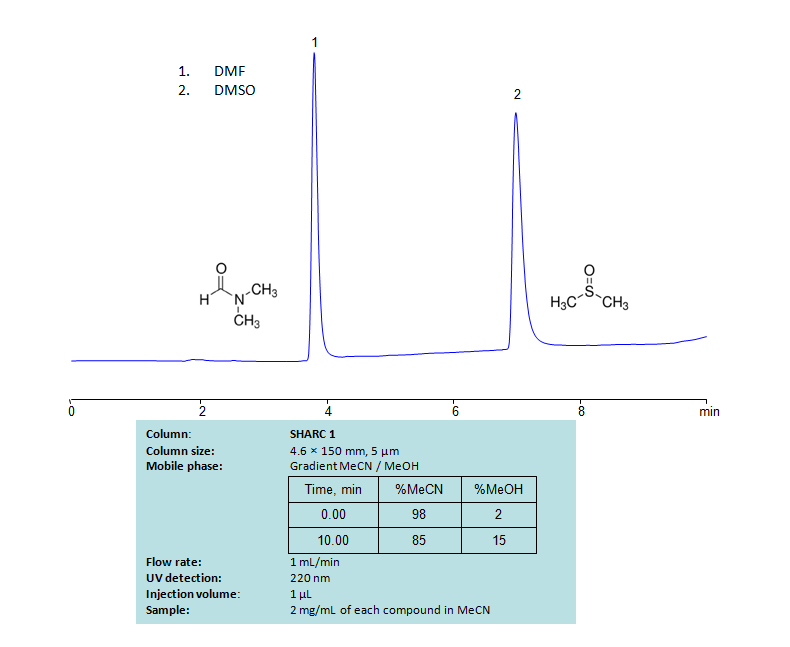
Dimethylformamide, DMF, is primarily used as an organic solvent and is miscible with water and most other organic liquids. Dimethyl sulfoxide (DMSO) is also a solvent. It can dissolve both polar and nonpolar compounds and is miscible with water and other organic liquids. Both compounds can be retained and separated using anhydrous (water-free) conditions using HPLC by SHARC 1 column, which uses hydrogen-bonding as a separation mechanism. The method uses a gradient of acetonitrile (ACN) and methanol (MeOH) mobile phase without the need for a buffer. UV detection used at 220nm.
| Column | SHARC 1, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 98-85 %, 10 min |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 220 nm |
| Class of Compounds |
Drug, Basic, Hydrophobic, Ionizable, Zwitterionic |
| Analyzing Compounds | DMF (Dimethylformamide), DMSO (Dimethyl sulfoxide) |
Application Column
SHARC 1
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
DMSO (Dimethyl sulfoxide)