| CAS Number | 37517-28-5 |
|---|---|
| Molecular Formula | C22H43N5O13 |
| Molecular Weight | 585.608 |
| InChI Key | LKCWBDHBTVXHDL-RMDFUYIESA-N |
| LogP | -4.09 |
| Synonyms |
|
Applications:
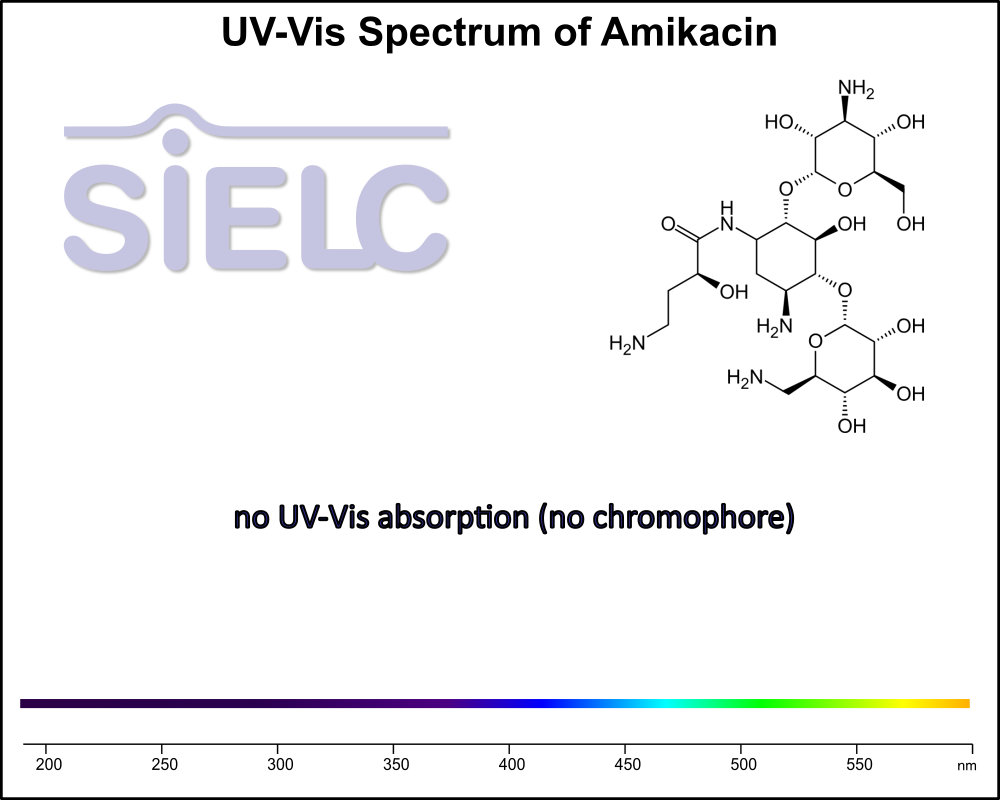
Uv-Vis Spectrum of Amikacin
January 13, 2026
If you are looking for optimized HPLC method to analyze Amikacin check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method of Amikacin on Obelisc R Column
August 22, 2008
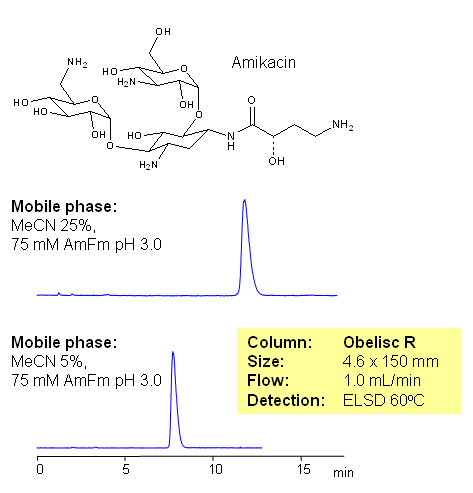
Amikacin is an aminoglycoside antibiotic used to treat different types of bacterial infections. Most of aminoglycoside antibiotic are very polar compounds containing several amino groups and sugar fragments. Such compounds are not retained on reverse phase column without ion-pairing reagents. In this application Amikacin is retained on Obelisc R column without ion-pairing reagent. Retention is contributed to mixed-mode mechanism and can be adjusted by buffer pH and buffer concentration. Amount of organic modifier (acetonitrile, tetrahydrophuran, etc.) can be used for fine tuning and improvement of peak shape. Other amino sugars can be analyzed using mixed-mode stationary phases with ELSD, LC/MS and prep chromatography compatible mobile phases.
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select options