| CAS Number | 113-92-8 |
|---|---|
| Molecular Formula | C20H23ClN2O4 |
| Molecular Weight | 390.860 |
| InChI Key | DBAKFASWICGISY-BTJKTKAUSA-N |
| LogP | 3.11 |
| Synonyms |
|
Applications:
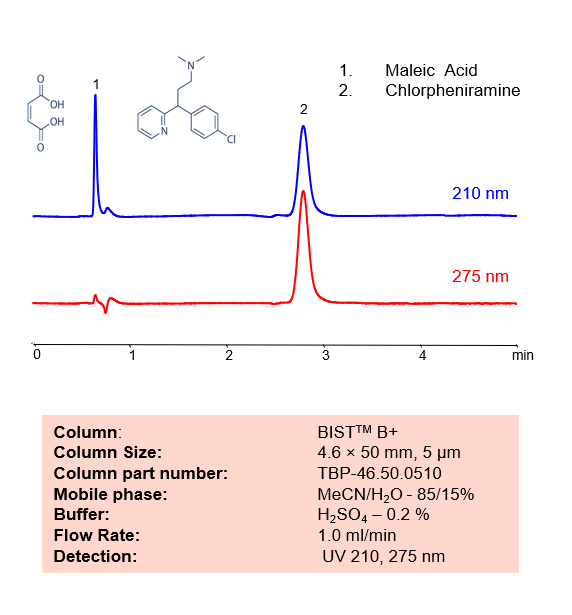
HPLC Method for Analysis of Chlorpheniramine Maleate on BIST B+
November 30, 2022
HPLC Method for Analysis of Chlorpheniramine Maleate on BIST B+ by SIELC Technologies.
Chlorpheniramine Maleate, also known as Chlorphenamine, is a popular antihistamine used to provide relief from the symptoms of allergies, hay fever, and the common cold with the chemical formula C16H19ClN2. It works through blocking histamine H1 receptor. It is said to produce less sedation than other first-generation histamines. Other side effects include drowsiness, dizziness, confusion, constipation, nausea, and more.
Using SIELC’s newly introduced BIST™ method, Chlorpheniramine Maleate, which separates in water, can be retained on a positively-charged anion-exchange BIST B+ column. There are two keys to this retention method: 1) a multi-charged, negative buffer, such as Sulfuric acid (H2SO4), which acts as a bridge, linking the positively-charged analytes to the positively-charged column surface and 2) a mobile phase consisting mostly of organic solvent (such as MeCN) to minimize the formation of a solvation layer around the charged analytes. Using this new and unique analysis method, Chlorpheniramine maleate can be separated, retained, and UV detected at 210 nm and 275 nm.
Condition
| Column | BIST B+, 4.6 x 50 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 85% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210, 275 nm |
| Peak Retention Time | 2.9 min |
Description
| Class of Compounds | Drug |
| Analyzing Compounds | Chlorpheniramine maleate |
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 50 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

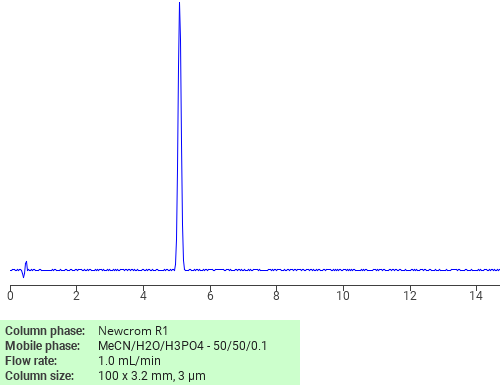
Separation of Chlorpheniramine maleate on Newcrom R1 HPLC column
February 16, 2018
Chlorpheniramine maleate can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options