| CAS Number | 16506-27-7 |
|---|---|
| Molecular Formula | C16H21Cl2N3O2 |
| Molecular Weight | 358.3 |
| InChI Key | YTKUWDBFDASYHO-UHFFFAOYSA-N |
| LogP | 2.9 |
| Synonyms |
|
Applications:
HPLC Analysis of Bendamustine and Related Impurities
July 10, 2012
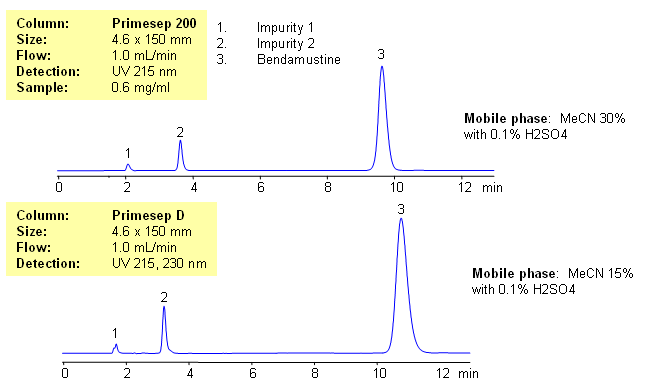
Application Notes: Bendamustine is a hydrophobic and basic drug, classified as nitrogen mustard. Two approaches were used to separate bendamustine from related impurities. In one approach, a Primesep 200 HPLC column was used in reversed-phase cation-exchange mode. Primesep D column was also used in reversed-phase cation-exclusion mode. Both methods are alternative to each other. Both can be used to quantify bendamustine in various sample matrices (drug composition, blood, plasma, urine, etc.). Sample preparation is required in the case of biofluids using the Primesep 200 column. Primesep D can be used for direct analysis of bendamustine in blood, plasma and urine. Proteins in the biofuids repel from stationary phase. Sulfuric acid in the mobile phase can be replaced with ammonium formate or acetate for LC/MS and with TFA for ELSD/CAD.
Application Columns: Primesep 200, Primesep D
Application compounds: bendamustine and related impurities
Detection technique: UV, LC/MS, ELSD/CAD
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 30% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 0.6 ml/min |
| Detection | UV, 215 nm |
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 15% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Drug, Chemotherapy, Hydrophilic, Ionizable |
| Analyzing Compounds | Bendamustine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsUV Detection

HPLC Analysis of Bendamustine and Related Impurities on Primesep 200 Column
June 12, 2002

Application Notes: Bendamustine is a hydrophobic and basic drug, classified as nitrogen mustard. Two approaches were used to separate bendamustine from related impurities. In one approach, a Primesep 200 HPLC column was used in reversed-phase cation-exchange mode. Primesep D column was also used in reversed-phase cation-exclusion mode. Both methods are alternative to each other. Both can be used to quantify bendamustine in various sample matrices (drug composition, blood, plasma, urine, etc.). Sample preparation is required in the case of biofluids using the Primesep 200 column. Primesep D can be used for direct analysis of bendamustine in blood, plasma and urine. Proteins in the biofuids repel from stationary phase. Sulfuric acid in the mobile phase can be replaced with ammonium formate or acetate for LC/MS and with TFA for ELSD/CAD.
Application Columns: Primesep 200, Primesep D
Application compounds: bendamustine and related impurities
Detection technique: UV, LC/MS, ELSD/CAD
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 30% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 0.6 ml/min |
| Detection | UV, 215 nm |
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 15% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Drug, Chemotherapy, Hydrophilic, Ionizable |
| Analyzing Compounds | Bendamustine |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended



