| CAS Number | 5192-03-0 |
|---|---|
| Molecular Formula | C8H8N2 |
| Molecular Weight | 132.167 |
| InChI Key | ZCBIFHNDZBSCEP-UHFFFAOYSA-N |
| LogP | 0.7 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of 5-Aminoindol on Primesep 100 Column
July 30, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of 5-Aminoindole on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of 5-Aminoindole
5-Aminoindole is an organic compound with the chemical formula C8H8N2. It is a derivative of indole and features an amino group at the 5-position.
Applications:
Pharmaceuticals: It can serve as a building block in the synthesis of various pharmaceutical compounds.
Research: Used in studies related to indole derivatives, which are important in medicinal chemistry.
5-Aminoindole be retained and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV 200 nm
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN – 55% |
| Buffer | H2SO4 -0.05% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Samples | 1.0 mg/ml MeCN/H2O – 50/50% |
| Injection volume | 1 µl |
| LOD* | 30 ppb (200 nm) |
| Class of Compounds | Pyridines |
| Analyzing Compounds | 5-Aminoindole |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A

Separation of Model Compounds in Reversed-Phase and Mixed-Mode
April 25, 2019
Separation type: Liquid Chromatography Mixed-mode
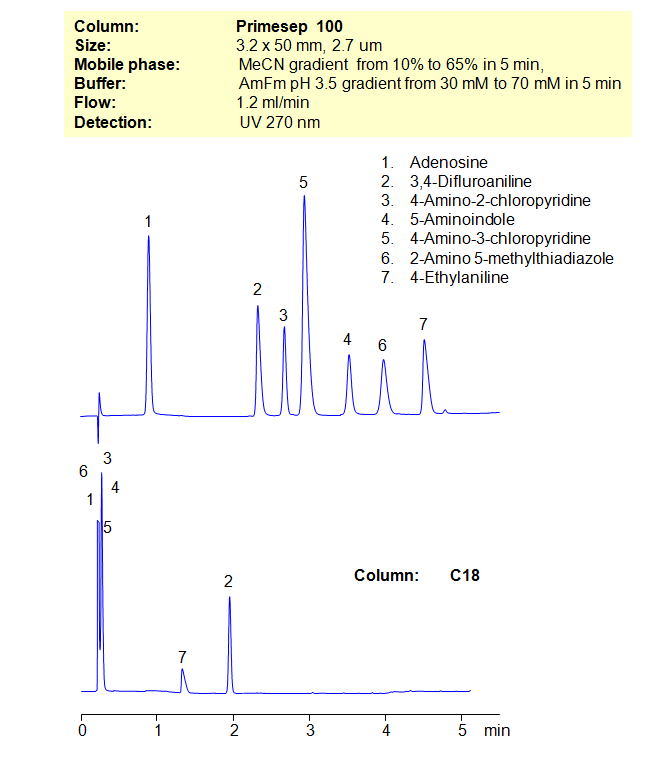
Many compounds are difficult, if not impossible, to separate on reverse-phase columns in HPLC. Other compounds cannot be separated on ion-exchange columns. That’s where the mixed-mode columns come in. By using a stationary phase with both hydrophobic and ion-exchange properties, allows the chromatographer to have additional controls over separation conditions. Here, we demonstrate the separation of compounds that can’t be achieved on a C18 column. By using both an organic gradient and buffer gradient of ammonium formate (AmFm), we can separate structurally similar compounds that can’t be separated on a reverse-phase column alone.
| Column | Primesep 100, 3,2×50 mm, 2,7 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-60%, 5 min |
| Buffer | Gradient AmFm pH 3.5- 30 – 70 mM, 5 min |
| Flow Rate | 1.2 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Basic, Hydrophilic, Hydrophobic, Ionizable. |
| Analyzing Compounds | Adenosine, 3,4-Difluroaniline, 4-Amino-2-chloropyridine, 5-Aminoindole, 4-Amino-3-chloropyridine, 2-Amino 5-methylthiadiazole, 4-Ethylaniline |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2-Amino-5-methyl-thiazole
3,4-Difluoroaniline
4-Amino-2-Chloropyridine
4-Amino-3-Chloropyridine
4-Ethylaniline
5-Aminoindole
Adenosine