| CAS Number | 116355-83-0 |
|---|---|
| Molecular Formula | C34H59NO15 |
| Molecular Weight | 721.8 |
| InChI Key | UVBUBMSSQKOIBE-DSLOAKGESA-N |
| LogP | -0.5 |
| Synonyms |
|
Applications:
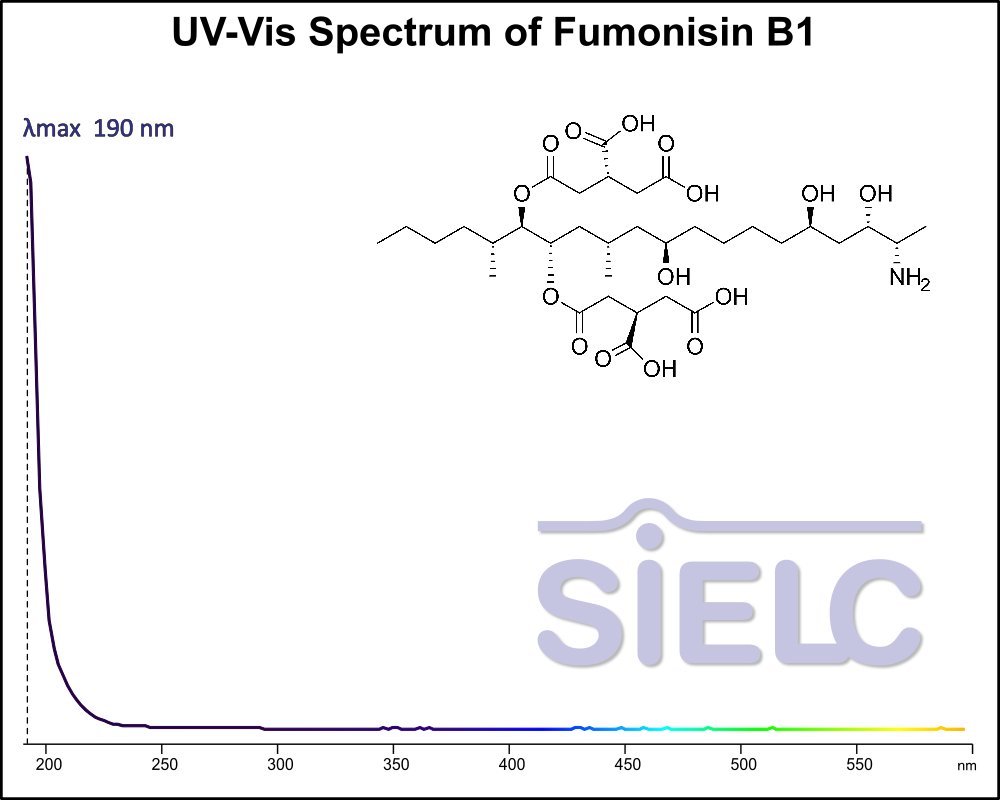
Uv-Vis Spectrum of Fumonisin B1
January 26, 2026
If you are looking for optimized HPLC method to analyze Fumonisin b1 check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Analysis of Fumonisin B1 on Primesep 100 Column
October 6, 2023
HPLC Method for Analysis of Fumonisin b1 on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Fumonisin b1
Fumonisin B1 is a type of mycotoxin produced by Fusarium molds, particularly Fusarium verticillioides and Fusarium proliferatum. These molds commonly infect corn and other crops, and through these, fumonisin B1 can enter the food chain. This toxin is found worldwide, and it’s especially a concern in areas where corn is a staple food.
Fumonisin B1 is a notable mycotoxin due to its stability, widespread occurrence, and potential to cause harm to animals and humans. Monitoring and managing levels of this toxin in food products is essential to safeguard public and animal health.
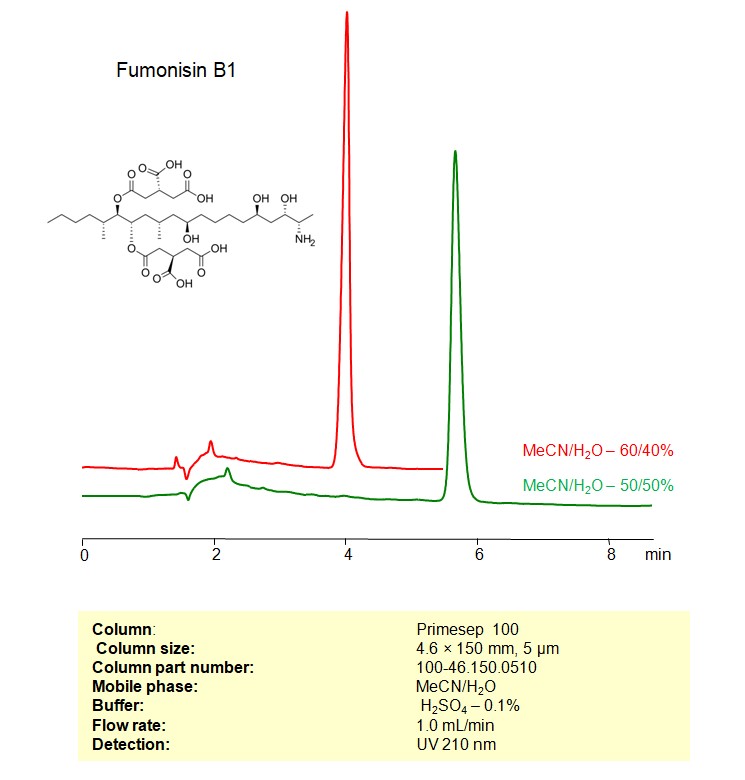
Fumonisin B1 can be retained, and analyzed on a Primesep 100 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid as a buffer. This analysis method can be detected using UV at 210 nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 -0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Class of Compounds | Drug |
| Analyzing Compounds | Fumonisin b1 |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended


