| CAS Number | 99-94-5 |
|---|---|
| Molecular Formula | C8H8O2 |
| Molecular Weight | 136.150 |
| InChI Key | LPNBBFKOUUSUDB-UHFFFAOYSA-N |
| LogP | 2.27 |
| Synonyms |
|
Applications:
Separation of Phthalic Acids and Related Impurities
July 2, 2013
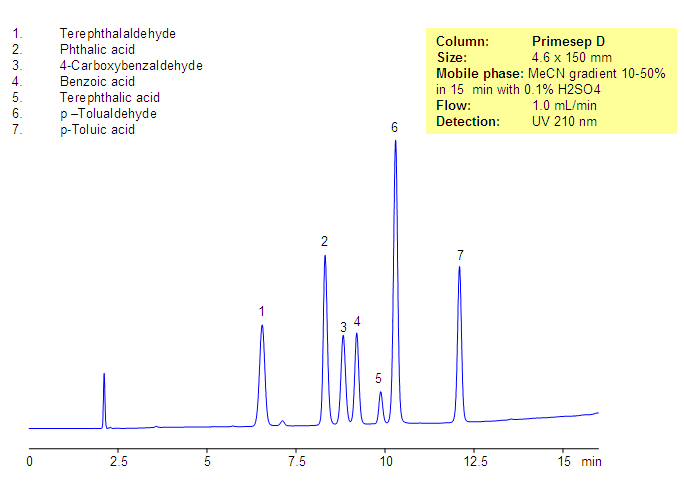
Phthalic acid, phthalic acid isomers, and related products present in the production of phthalic acid were separated on the Primesep D column, based on reversed-phase and in-exchange mechanisms. Neutral, hydrophobic compounds of the phthalic acid production are retained by a reversed-phase mechanism, and phthalic acid and other acidic compounds are retained by a combination of reversed-phase and anion-exchange mechanisms. Resolution and selectivity of this separation can be modified by varying the amount of acetonitrile, buffer concentrations, and buffer pH. This method can be used for monitoring the production cycle of phthalic acid and related impurities.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-50%, 15 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Terephthalaldehyde, Phthalic acid, 4-Carboxybenzaldehyde, Benzoic acid, Terephthalic acid, p –Tolualdehyde, p-Toluic acid |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzoic Acid
Phthalic Acid
Terephthalaldehyde
Terephthalic Acid
p-Tolualdehyde
p-Toluic Acid