| CAS Number | 1448-36-8 |
|---|---|
| Molecular Formula | C25H42O5 |
| Molecular Weight | 422.6 |
| InChI Key | DLYVTEULDNMQAR-SRNOMOOLSA-N |
| LogP | 3.9 |
| Synonyms |
|
Applications:
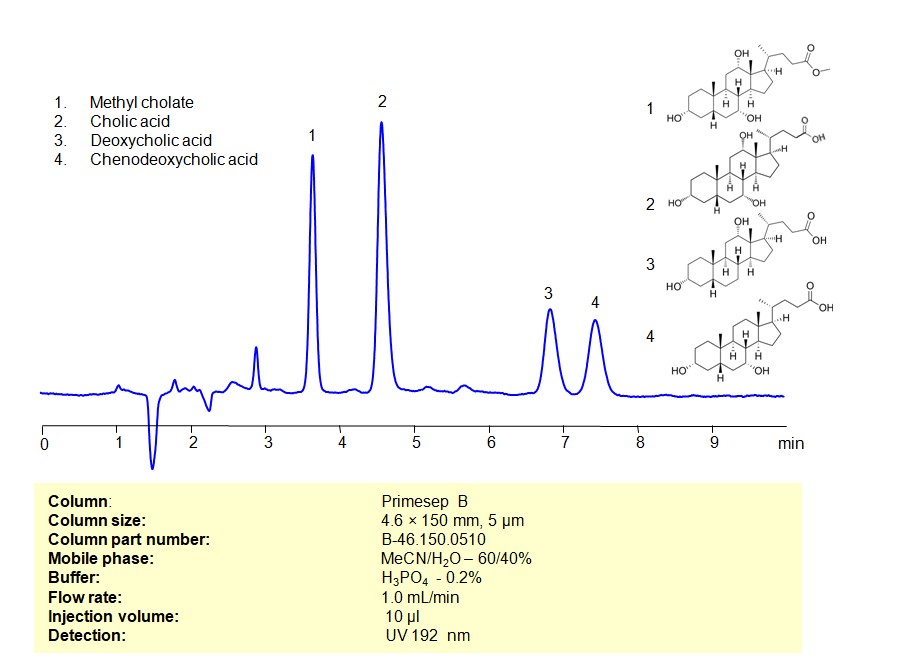
HPLC Method for Separation of Bile acids (Methyl cholate, Cholic acid, Deoxycholic acid, Chenodeoxycholic acid) on Primesep B Column
January 24, 2024
HPLC Method for Analysis of Methyl cholate, Cholic acid, Deoxycholic acid, Chenodeoxycholic acid on Primesep B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Methyl cholate, Cholic acid, Deoxycholic acid, Chenodeoxycholic acid
Bile acids are a class of amphipathic molecules derived from cholesterol that play a crucial role in the digestion and absorption of dietary fats. They are synthesized in the liver and released into the small intestine during digestion.
- Cholic Acid:
- Cholic acid is a primary bile acid.
- It is synthesized in the liver from cholesterol.
- Cholic acid contributes to the emulsification and digestion of fats in the small intestine.
- Deoxycholic Acid:
- Deoxycholic acid is a secondary bile acid.
- It is formed by bacterial action in the colon on cholic acid.
- Deoxycholic acid also aids in the digestion and absorption of fats.
- Chenodeoxycholic Acid:
- Chenodeoxycholic acid is another primary bile acid.
- It is synthesized in the liver.
- Like cholic acid, it participates in the emulsification of fats.
- Methyl Cholate:
- Methyl cholate is a derivative of cholic acid.
- It is formed by adding a methyl group to cholic acid.
- Bile acids, including methyl cholate, contribute to the solubilization of lipids.
These bile acids are part of the bile acid pool, which undergoes enterohepatic circulation—being released into the small intestine, reabsorbed in the terminal ileum, and returned to the liver. Bile acids also play a role in cholesterol metabolism and act as signaling molecules.
Their chemical structures, amphipathic nature, and interactions with lipids are essential for their physiological functions in the digestive process. The balance of bile acids in the body is crucial for proper digestion and absorption of dietary fats.
Bile acids can be retained, separated, and analyzed using a Primesep B mixed-mode stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and sulfuric acid as a buffer. Detection is achieved using UV at 192 nm
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O – 60/40% |
| Buffer | H3PO4 -0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 192 nm |
| Samples | 2 mg/mL in MeCN/H2O – 50/50% |
| Injection volume | 10 µl |
| LOD* | 0.2 ppm |
| Class of Compounds | bile acids |
| Analyzing Compounds | Methyl cholate, Cholic acid, Deoxycholic acid, Chenodeoxycholic acid |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Cholic acid
Deoxycholic acid
Methyl cholate

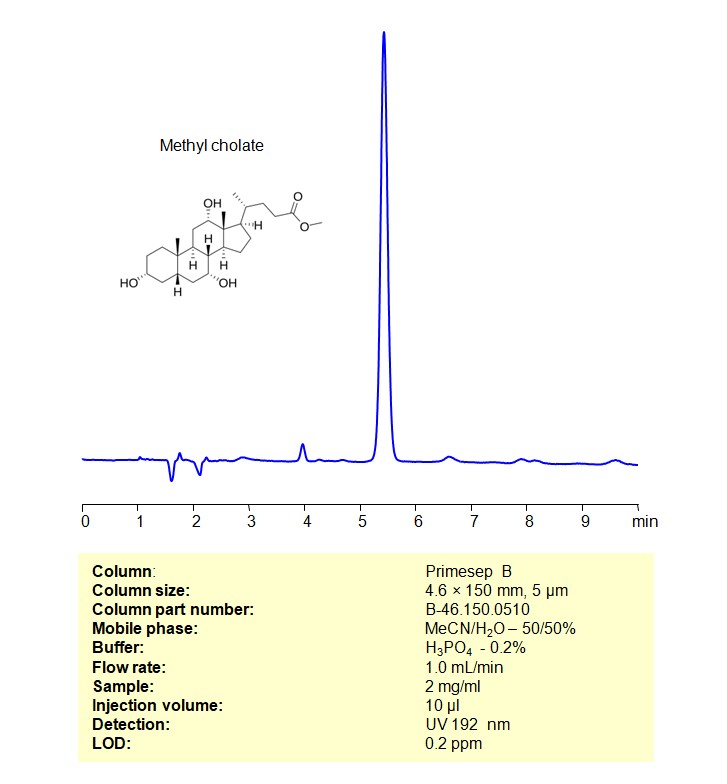
HPLC Method for Analysis of Methyl cholate on Primesep B Column
January 23, 2024
HPLC Method for Analysis of Methyl cholate on Primesep B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Methyl cholate
Methyl cholate is a compound that belongs to the class of bile acids. Bile acids are substances that are produced by the liver and play a crucial role in the digestion and absorption of dietary fats. Cholate is a primary bile acid, and when methylated, it forms methyl cholate.
- Methyl cholate is derived from cholic acid, one of the primary bile acids. The addition of a methyl group to cholic acid results in the formation of methyl cholate.
- Bile acids, including cholic acid and its derivatives like methyl cholate, aid in the emulsification and digestion of fats in the small intestine. They also play a role in the absorption of fat-soluble vitamins.
- Bile acids, including methyl cholate, undergo complex metabolic pathways in the liver and are released into the small intestine during digestion to aid in the breakdown of fats.
It’s important to note that bile acids have physiological roles beyond digestion, including the regulation of cholesterol metabolism and acting as signaling molecules.
Methyl cholate can be retained and analyzed using a Primesep B mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV at 192 nm
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | H3PO4 -0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 192 nm |
| Samples | 2 mg/mL in MeCN/H2O – 50/50% |
| Injection volume | 10 µl |
| LOD* | 0.2 ppm |
| Class of Compounds | bile acids |
| Analyzing Compounds | Methyl cholate |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A


