NEW SEPARATION APROACH
Many organic molecules of medical, pharmaceutical, industrial, and environmental interest can exist in an ionic form at specific pH levels. One such example is oligonucleotides (OGNs), which are important group of negatively charged molecules used in a variety of applications including medicine, research, and forensics.
Antisense Oligonucleotides (ASOs) such as Nusinersen and Mipomersen are pharmaceuticals with oligonucleotide chemistry. Liquid chromatography (LC) separation of this class of molecules is an important step in their production, characterization, and purification.
There are several separation modes based on different properties of the oligonucleotide molecules. This separation occurs on the boundary of solid stationary phase with specifically modified surface and liquid mobile phase.
We recently reported a new mode of chromatography which we named Bridge Ion Separation Technique (BIST™). This technique was developed in order to obtain better separation control when working with multicharged molecules. Oligonucleotides are exactly the type of molecules that can be separated by this new separation mode.
This method requires some amount of organic modifier in the mobile phase and a double-charged positive ion (TMEDA – tetramethylethylenediamine) as a buffer component (Figure 1).
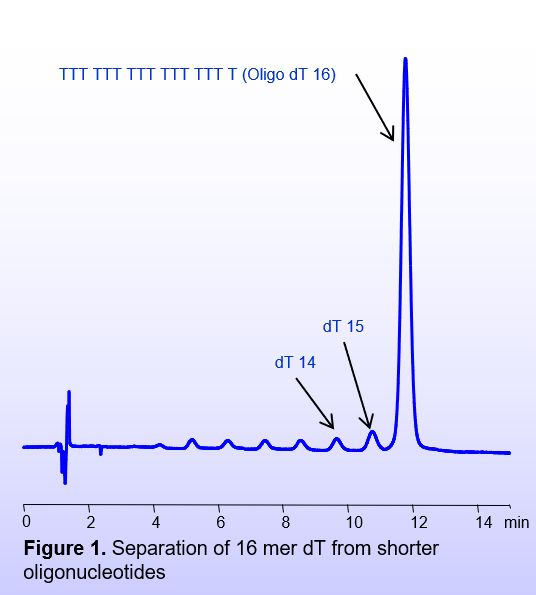
Column: BIST™ AC
Column size: 4.6 × 100 mm, 5 µm
Part number: TA-46.100.0510.C
Mobile phase: Gradient MeCN – 50 -35%, 15 min
Buffer: TMEDA acetate – 20 mM, pH 4.0
Flow rate: 1.0 mL/min
Detection: UV 260 nm
See full method details here
BIST™ works differently. Three conditions need to be met:
– A double-charged buffer ion present in the mobile phase
– Buffer’s double-charged ions should be opposite in charge to that of the stationary phase surface
– Reduced water in the mobile phase to control ion solvation
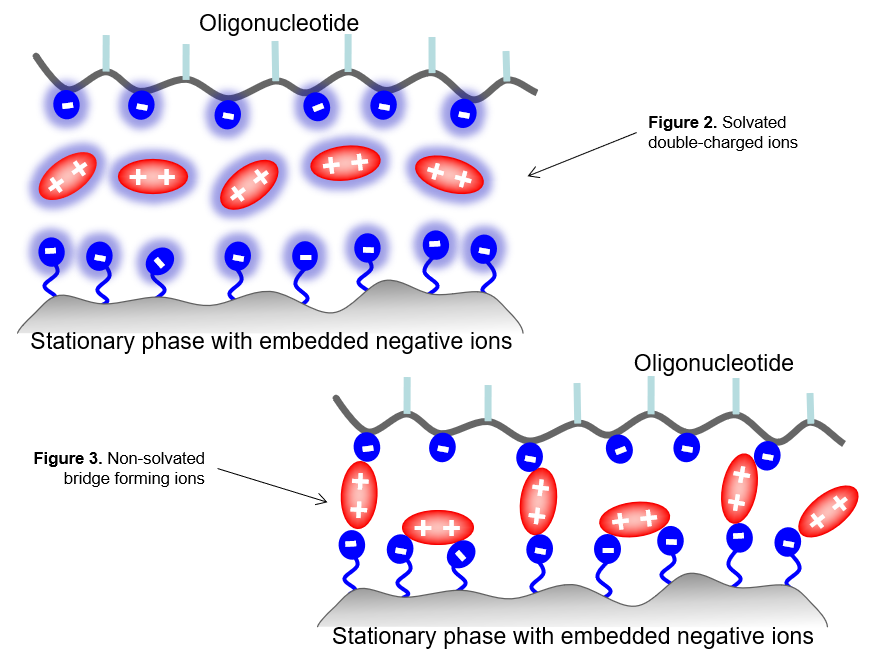
The above mechanism has been proposed and supported by multiple experiments. When fully solvated ions interact with a stationary phase in an aqueous medium, the only retaining force can be an electrostatic attraction between the oppositely charged ions of the analytes and the stationary phase (Figure 2). However, if the ions are not fully solvated, they are able to form stable ion-pairs. Moreover, if the buffer ions have a double charge, they can invert the net surface charge while they are retained by the stationary phase.
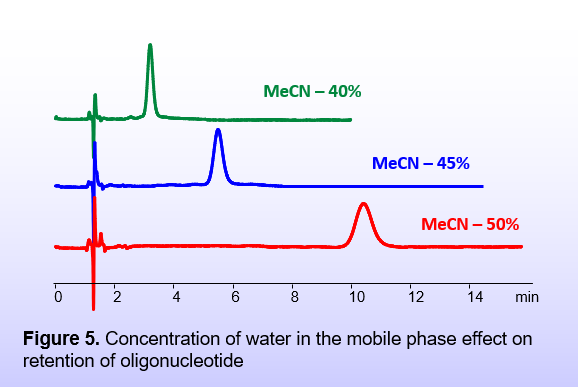
The amount of water in the mobile phase is also critically important in controlling the solvation level of the ions. Less solvation leads to ions being more tightly bound, resulting in a stronger interaction between the analytes and the stationary phase (Figure 5). For instance, a negatively charged surface can become positively charged in the presence of double-charged non-solvated positive ions of the mobile phase (Figure 3). This creates an opportunity to retain analytes of the same charge sign as the surface.
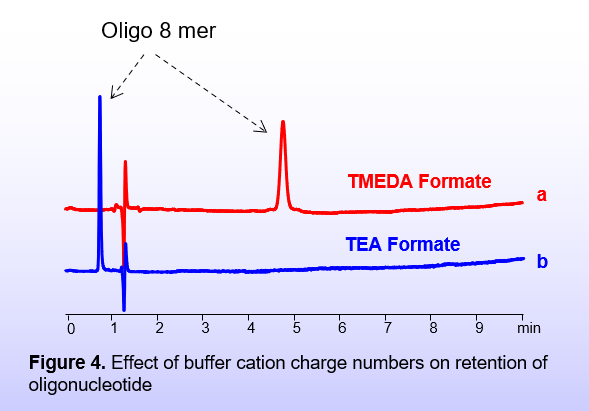
This mechanism is not possible if the buffer does not provide double-charged ions. This example on the left (Figure 4) demonstrates that when the buffer cation decreases from 2+ (TMEDA) to 1+ (TEA), the interaction of the oligonucleotide changes from retention (4a) to repulsion (4b).
Column: BIST™ AC
Column size: 4.6 × 100 mm, 5 µm
Column part number: TA-46.100.0510.C
Mobile phase: Gradient MeCN – 50-5%, 10 min
Buffer: pH 4.0 – 20 mM
Flow rate: 1.0 mL/min
Detection: UV 260 nm
The amount of water in the mobile phase is critically important to controlling the solvation level of the ions. The lower the solvation, the better the bridge formation of the analytes with the stationary phase (Figure 5).
Column: BIST™ AC
Column size: 4.6 × 100 mm, 5 µm
Column part number: TA-46.100.0510.C
Mobile phase: Gradient MeCN – 50-5%, 10 min
Buffer: pH 4.0 – 20 mM
Flow rate: 1.0 mL/min
Detection: UV 260 nm
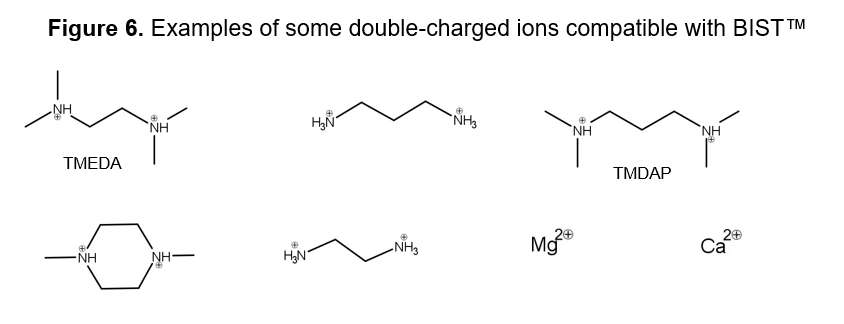
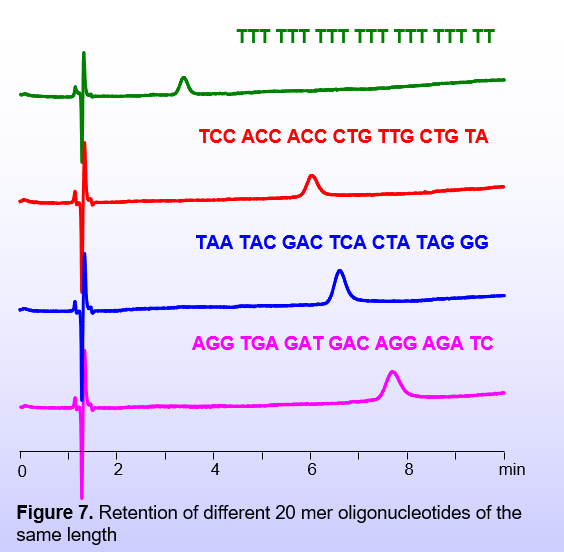
The degree of retention in BIST™ mode depends not only on the number of charges in the chain but also on the chain’s composition (Figure 7). Typically, an increase in the number of T (Thymine) units lowers the retention of the oligomers. This is attributed to the stronger basic property of Thymine when compared to other nucleobases, compensating to some degree for the acidic property of the oligonucleotide.
Column: BIST AC
Column size: 4.6 × 100 mm, 5 µm
Column part number: TA-46.50.0510.C
Mobile phase: Gradient MeCN – 40-5%, 10 min
Buffer: TMEDA Formate – 20 mM, pH 4.0
Flow rate: 1 mL/min
Detection: UV 260 nm
See full method details here
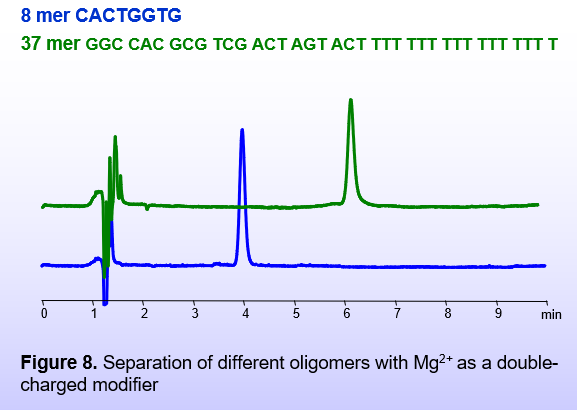
The BIST™ method can use various modifiers with double positive charges (Figure 6). Magnesium acetate can be a convenient buffer modifier of the mobile phase if a volatile mobile phase is not required (Figure 8).
Column: BIST™ AC
Column size: 4.6 × 100 mm, 5 µm
Column part number: TA-46.100.0510.C
Mobile phase: Gradient MeCN – 50-5%, 10 min
Buffer: Mg Acetate – 20 mM, pH 4.0
Flow rate: 1.0 mL/min
Detection: UV 260 nm
CONCLUSION
One important advantage of BIST™ when compared to ion-exchange separation is the low buffer concentration requirements for multicharged analytes. Typically, a buffer concentration is 20 mM or less. There is insignificant change in the retention and selectivity observed if the buffer concentration is increased above 20 mM.
When compared with reverse-phase separation of oligonucleotides in presence of ion-pairing reagents, one important benefit is a short column equilibration time with gradient separation.
Moreover, the introduction of a new separation mode such as BIST™ can open up new possibilities for tackling complex analytical or preparative challenges.

