| CAS Number | 98-10-2 |
|---|---|
| Molecular Formula | C6H7NO2S |
| Molecular Weight | 157.191 |
| InChI Key | KHBQMWCZKVMBLN-UHFFFAOYSA-N |
| LogP | 0.31 |
| Synonyms |
|
Applications:
HPLC Separation of Benzenesulfonamide and Sulfanilamide
August 22, 2008
| Column | Newcrom А, 3.2×50 mm, 3 µm, 100A |
| Mobile Phase | MeOH/H2O – 80/20% |
| Buffer | H2SO4 – 0.005% |
| Flow Rate | 0.5 ml/min |
| Detection | 275 nm |
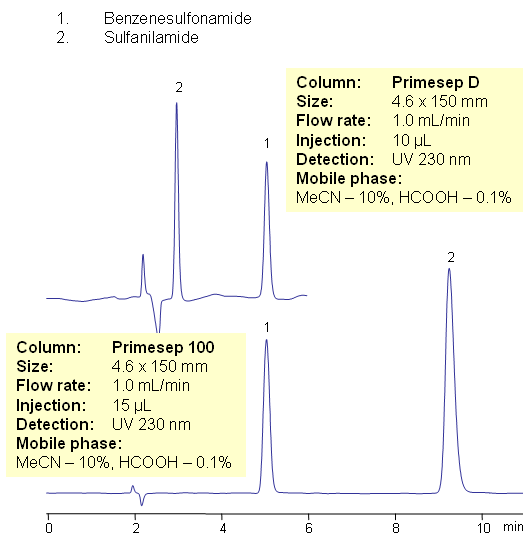
| Column | Primesep D, , Primesep 100 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | Formic Acid – 0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 230 nm |
Both Benzenesulfonamide and sulfanilamide are organic antibacterial compounds differing only in the addition of an extra amino group in sulfanilamide. The closely related compounds can be separated on a mixed-mode Primesep D column by hydrophobic and anion-exchange mechanisms or Primesep 100 column by hydrophobic and cation-exchange mechanisms with the elution order reversed using identical mobile phases. UV Detection at 230nm.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsSulfanilamide

HPLC Separation of Aromatic Sulfonamides and Hydrozine
December 6, 2007
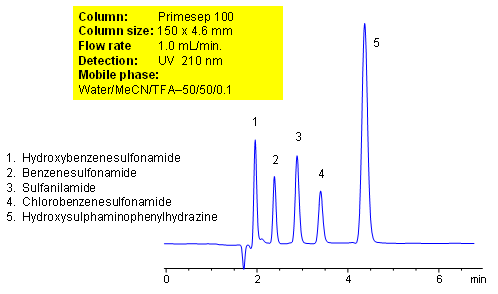
Chlorobenzenesulfonamide
Hydroxybenzenesulfonamide
Hydroxysulphaminophenylhydrazine
Sulfanilamide

Separation of Sulfonamides and Phenylhydrazine by Mixed-Mode HPLC
May 5, 2005
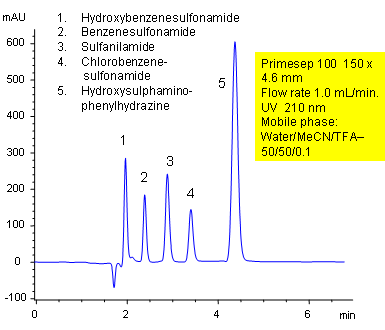
Primesep 100 separates a mixture of sulfonamides (benzenesulfonamide, chlorobenzenesulfonamide, hydroxybenzenesulfonamide) and hydroxysulphaminophenylhydrazine by a mixture of polar and hydrophobic interactions. The stationary phase’s hydrophobic functionality provides a reversed-phase mechanism while the embedded cation-exchange group provides polar interactions. The separation method uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm. This method is mass spec (LC/MS) and evaporative light scattering (ELSD) compatible.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsChlorobenzenesulfonamide
Hydroxybenzenesulfonamide
Hydroxysulphaminophenylhydrazine
Sulfanilamide
Sulfonamides