| CAS Number | 2243-47-2 |
|---|---|
| Molecular Formula | C12H11N |
| Molecular Weight | 169.227 |
| InChI Key | MUNOBADFTHUUFG-UHFFFAOYSA-N |
| LogP | 2.62 |
| Synonyms |
|
Applications:
HPLC Separation of Aminobiphenyls
April 10, 2005
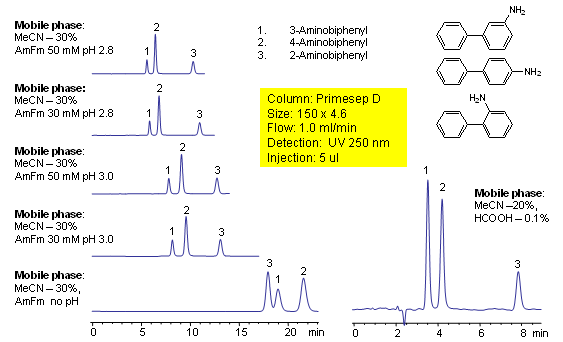
Aminobiphenyls consist of isomers that differ only in the location of a primary amine group on an aromatic ring. 2-, 3-, and 4-aminobiphenyl are separated on a Primesep D with good peak shape and a short retention time by a mixture of reversed-phase and ion-exclusion interactions. Peak order can be reversed for 2- and 3-aminobiphenyls by using ammonium formate buffer without pH adjustment. The HPLC separations use a mobile phase of water, acetonitrile (MeCN, ACN), ammonium formate buffer or acetic acid (HOAc) with UV detection at 250 nm.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | Formic Acid, AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Hydrophobic, Ionizable |
| Analyzing Compounds | 2-Aminobiphenyl, 4 -Aminobiphenyl, 3 -Aminobiphenyl |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options3-Aminobiphenyl
4-Aminobiphenyl