HPLC Method for Ferrous fumarate on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ferrous fumarate.
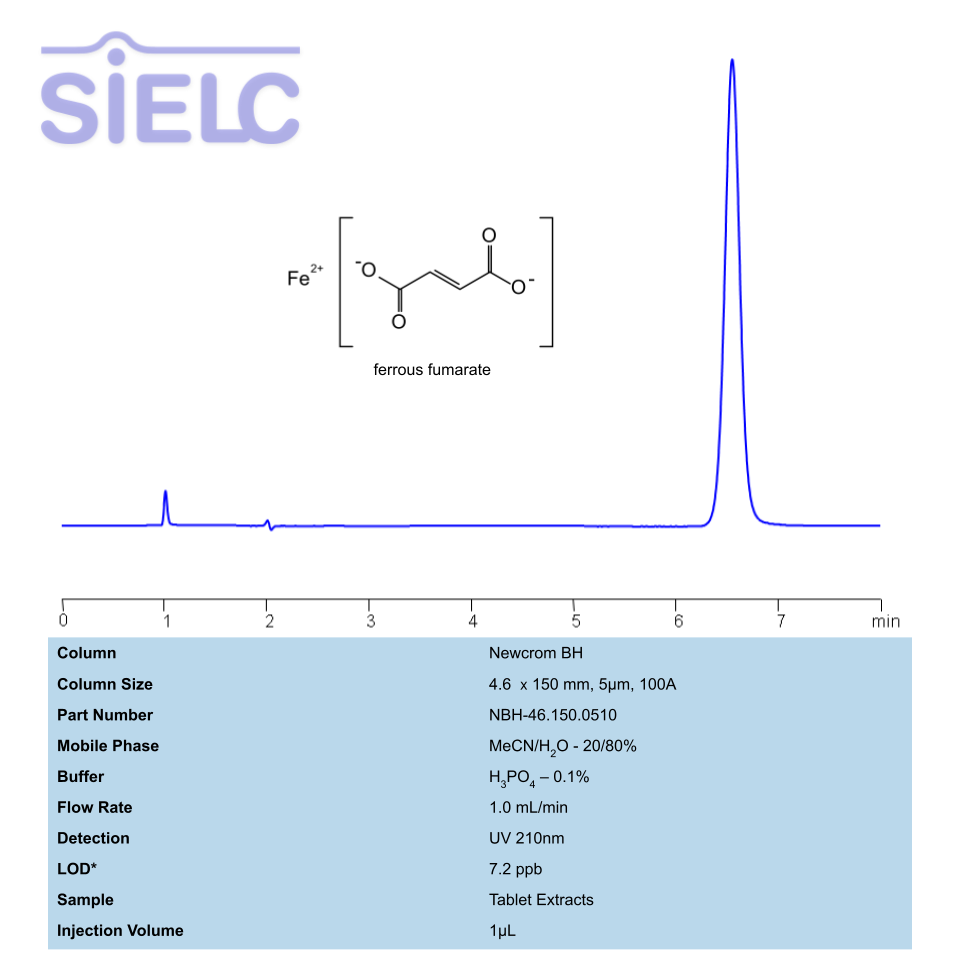
Ferrous Fumarate, also known as Iron(II) fumarate, is iron(II) salt of fumaric acid with the molecular formula C4H2FeO4. It is used as an iron supplement to prevent iron deficiency anemia. When mixed with potassium iodate, it is also used to address iodine deficiency.
Ferrous fumarate can be retained and analyzed using the Newcrom BH stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN). Detection is performed using UV.
Preparation of Fumaric Acid Standard Solutions
Stock Solution (1 mg/mL fumaric acid):
- Weigh exactly 1.0 mg of fumaric acid.
- Dissolve in 1.0 mL of deionized water.
- Shake thoroughly or vortex until completely dissolved
Dilution Series:
Prepare the following standard concentrations from the stock solution using deionized water as the diluent.
Preparation of Tablet Extracts (Ferrous Fumarate Tablets)
Each tablet contains ferrous fumarate, providing 25 mg of elemental iron, which is equivalent to approximately 51 mg of fumaric acid per approx. 314 mg tablet.
Extract (Target ~0.20 mg/mL fumaric acid):
- Weigh tablet(s) and crush to a fine powder.
- Accurately weigh an amount containing ~31.4 mg tablet(s) fine powder
- Transfer to a 25 mL volumetric flask.
- Add 2% H₂SO₄ in water and dilute to volume.
- Shake or mix thoroughly to dissolve.
- Filter to remove any undissolved excipients.
- Use directly for analysis.
Theoretical concentration: ~0.20 mg/mL
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 20% |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210nm |
| LOD* UV | 7.2 ppb |
| Class of Compounds | Salt |
| Analyzing Compounds | Ferrous fumarate |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended