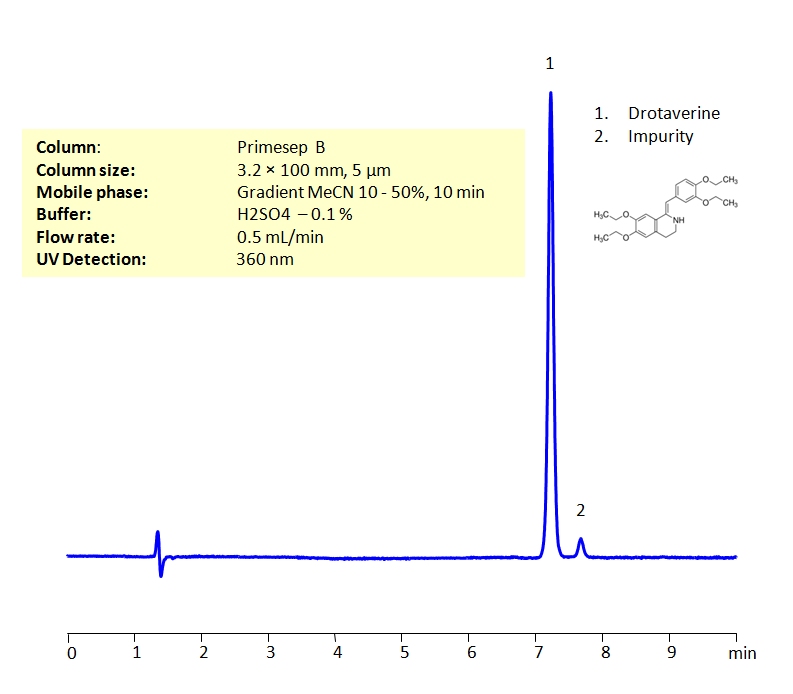
HPLC Method for Drotaverine on Primesep B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Drotaverine.
Drotaverine (known under the brand name No-Spa in Europe) is an antispasmodic commonly encountered in the hospital setting to increase cervical dilation during childbirth. It is also commonly used to alleviate gastrointestinal and genitourinary smooth muscle spasms. There have been issues with the drug being counterfeited or smuggled into countries that have yet to approve it, so having an HPLC method to detect the drug and seperate it from impurities and other unwanted compounds is a must for the pharmaceutical community. It’s chemical formula is C24H31NO4.
Drotaverine can be detected in the high UV regime. Using a Primesep B reverse-phase column and a mobile phase consisting of water and an acetonitrile (MeCN) gradient with a sulfuric acid (H2SO4) buffer, drotaverine can be retained, separated (from impurities and other unwanted compounds). This analysis method can be UV detected at 360 nm.
| Column | Primesep B, 3.2 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 10-50%, 10 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 245, 305, 360 nm |
| Class of Compounds | Drug |
| Analyzing Compounds | Drotaverine |
Application Column
Primesep B
Column Diameter: 3.2 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended