High Performance Liquid Chromatography (HPLC) Method for Analysis of Mirabegron on Primesep 500 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
Mirabegron is a medication used in the treatment of overactive bladder (OAB), a condition characterized by symptoms such as frequent urination, urgent need to urinate, and urinary incontinence.
Usage: It is prescribed for the symptomatic treatment of overactive bladder. Mirabegron helps in managing symptoms like urinary frequency, urgency, and incontinence.
Mirabegron offers an alternative to antimuscarinic drugs, which are another class of medications used for OAB but can have side effects like dry mouth and constipation. Mirabegron generally has a lower risk of these anticholinergic side effects.
Mirabegron represents a significant advancement in the treatment of overactive bladder, offering benefits in terms of efficacy and a favorable side effect profile compared to traditional antimuscarinic agents.
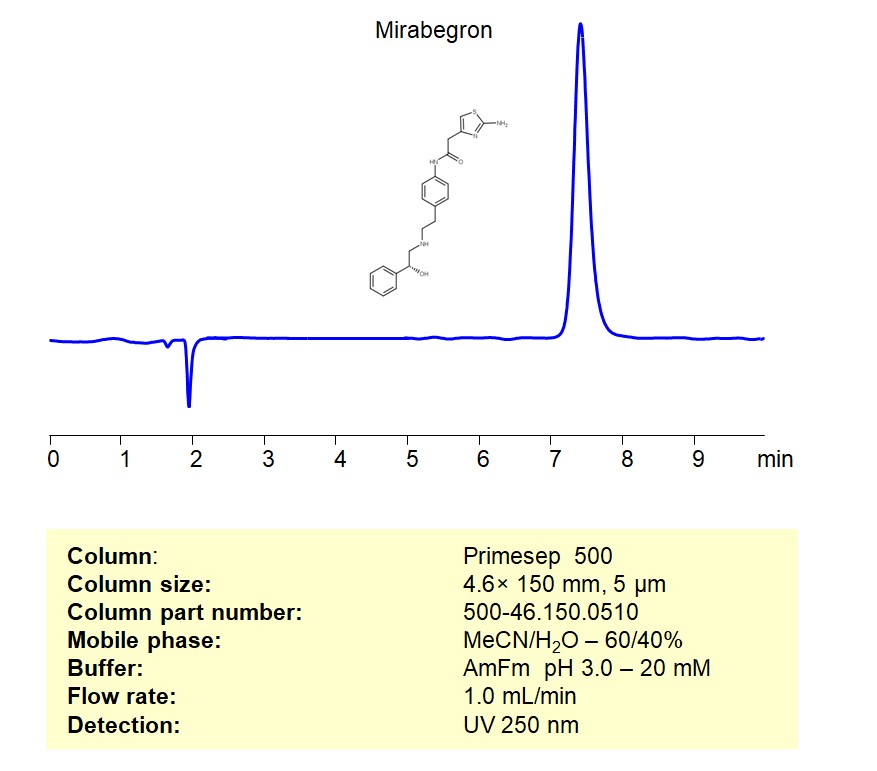
Mirabegron can be retained, and analyzed on a Primesep 500 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a Ammonium formate as a buffer. This analysis method can be detected using UV at 250 nm.
High Performance Liquid Chromatography (HPLC) Method for Analyses of Mirabegron
Condition
| Column | Primesep 500, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 60/40% |
| Buffer | Ammonium formate pH 3.0 – 20 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
| Peak Retention Time | 7.26 min |
Description
| Class of Compounds | Drug |
| Analyzing Compounds | Mirabegron |
Application Column
Primesep 500
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended





