| CAS Number | 133-06-2 |
|---|---|
| Molecular Formula | C9H8Cl3NO2S |
| Molecular Weight | 300.580 |
| InChI Key | LDVVMCZRFWMZSG-UHFFFAOYSA-N |
| LogP | 2.80 |
| Synonyms |
|
Applications:
HPLC Separation of Captan on Obelisc R and Primesep 100 Columns
September 14, 2015
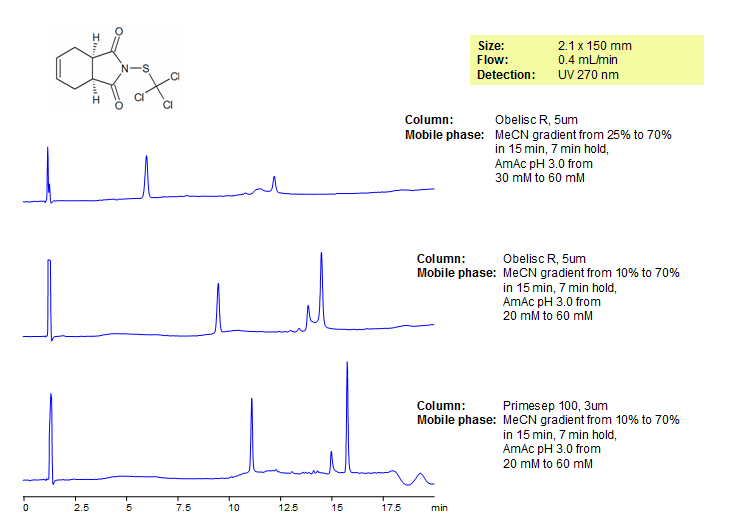
Captan is a fungicide that is typically combined with other pesticides. It is in the class of fungicides called phthalimide, and is used on a number of vegetables and ornamental plants. Captan reduces infections on the surface of plant material improving their appearance. The EURL-SRM (European Union Reference Laboratory – Single Residue Methods) included captan in a list of pesticides difficult to analyze by traditional multiresidue methods. We separated and analyzed captan using two mixed-mode columns with different modes of separation. Obelisc R has a long hydrophobic chain and multiple ion-pairing groups, and Primesep 100 contains acidic ion-pairing groups. Method is LC/MS compatible and can be use on many different pesticides.
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 25-70% |
| Buffer | Gradient AmAc pH 3.0- 30-60 mM |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-70% |
| Buffer | Gradient AmAc pH 3.0- 20-60 mM |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Column | Primesep 100, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-70% |
| Buffer | Gradient AmAc pH 3.0- 20-60 mM |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Insecticide, Pesticide, Hydrophobic, Ionizable |
| Analyzing Compounds | Captan |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsPrimesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options
Captan Retention on Obelisc R HPLC Column
August 24, 2015
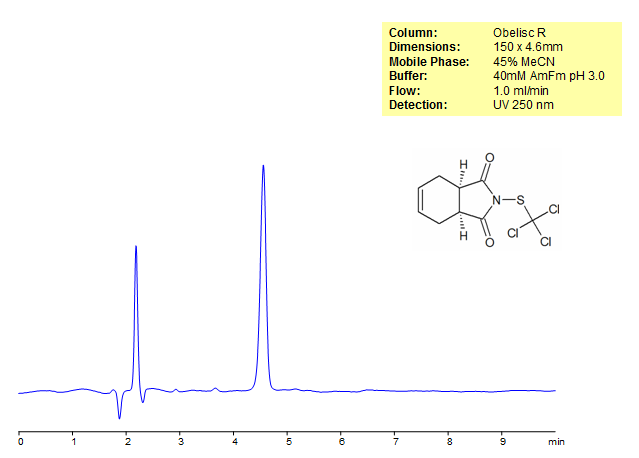
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 45% |
| Buffer | AmFm pH 3.0- 40 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Insecticide, Herbicide, Fungicide, Hydrophobic, Ionizable |
| Analyzing Compounds | Captan |
Application Column
Obelisc R
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A

Captan Separation on Obelisc and Primesep Mixed-Mode HPLC Columns
August 24, 2015
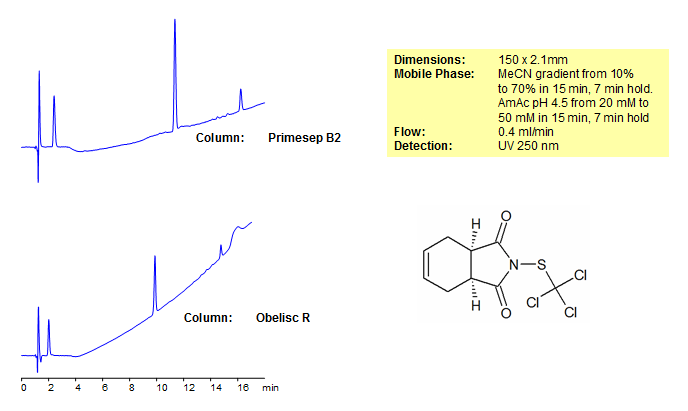
| Column | Primesep B2, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-70%, 15 min, 7 min hold |
| Buffer | Gradient AmAc pH 4,5- 20-50 mM, 15 min, 7 min hold |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 250 nm |
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 10-70%, 15 min, 7 min hold |
| Buffer | Gradient AmAc pH 4,5- 20-50 mM, 15 min, 7 min hold |
| Flow Rate | 0.4 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Insecticide, Herbicide, Fungicide, Hydrophobic, Ionizable |
| Analyzing Compounds | Captan |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsPrimesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options