| CAS Number | 5122-94-1 |
|---|---|
| Molecular Formula | C12H11BO2 |
| Molecular Weight | 198.030 |
| InChI Key | XPEIJWZLPWNNOK-UHFFFAOYSA-N |
| LogP | 2.91 |
| Synonyms |
|
Applications:
HPLC Separation of Aromatic Boronic Acids on Primesep P
July 8, 2011
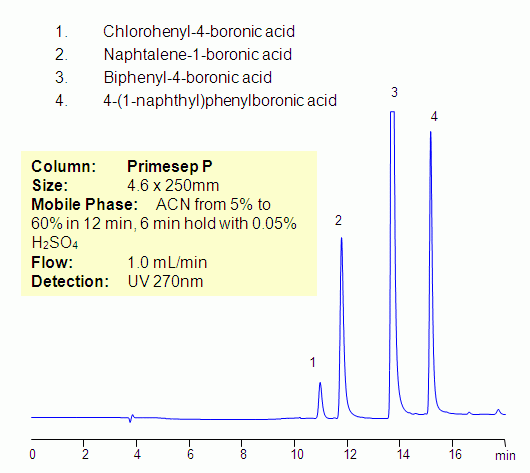
Boronic acid is alkyl, or aryl substituted, boric acid. Boronic acid can form complexes with sugars, amines, and amino acids. Four aromatic boronic acids were separated on a Primesep P mixed-mode reversed-phase cation-exchange column. Primesep P column also has an aromatic fragment which provides pi-pi interaction. Compounds can be monitored by UV, ELSD, CAD and LC/MS. Various mobile phases can be employed for HPLC analysis of boronic acids.
| Column | Primesep P, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophobic, Ionizable |
| Analyzing Compounds | Chlorohenyl-4-boronic acid, Naphtalene-1-boronic acid, Biphenyl-4-boronic acid, 4-(1-naphthyl)phenylboronic acid |
Application Column
Primesep P
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
4-Chlorophenylboronic acid
Biphenyl-4-boronic acid
Naphthalene-1-boronic acid