| CAS Number | 95-54-5 |
|---|---|
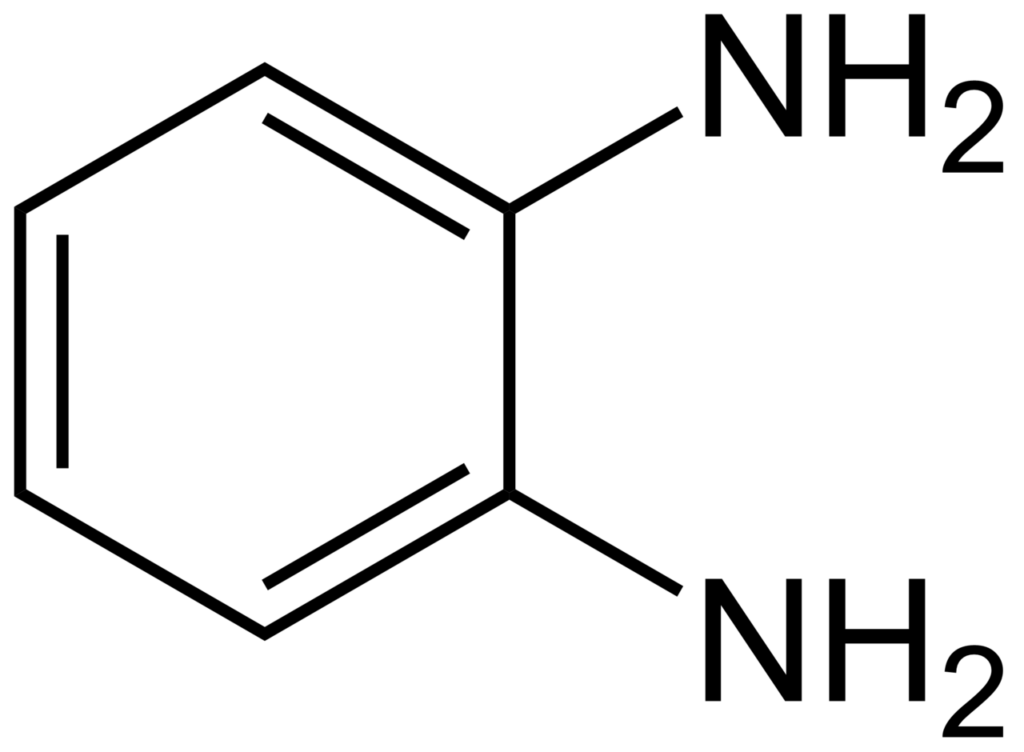
| Molecular Formula | C6H4 (NH2)2 |
| Molecular Weight | 1804.14 |
| InChI Key | GEYOCULIXLDCMW-UHFFFAOYSA-N |
| LogP | 0.1 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Isomers of Phenylenediamine on Primesep 100 Column
August 1, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of m-Phenylenediamine, o-Phenylenediamine, p-Phenylenediamine on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of m-Phenylenediamine, o-Phenylenediamine, p-Phenylenediamine
o-Phenylenediamine, m-Phenylenediamine, and p-Phenylenediamine are isomers of phenylenediamine, where the amino groups are attached to different positions on the benzene ring.
These compounds are important in various industrial applications due to their role as intermediates in the synthesis of other chemicals.
m-Phenylenediamine, o-Phenylenediamine, p-Phenylenediamine can be retained, separated and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV 200 nm
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN – 40% |
| Buffer | H2SO4 -0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Samples | 0.3 mg/ml in MeCN/H2O – 50/50% |
| Injection volume | 1 µl |
| LOD* | 10 ppb (200 nm) |
| Class of Compounds | Aromatic amines |
| Analyzing Compounds | m-Phenylenediamine, o-Phenylenediamine, p-Phenylenediamine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
o-Phenylenediamine
p-Phenylenediamine


