HPLC Method for TFA (Trifluoroacetic Acid) on Newcrom BH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of TFA (Trifluoroacetic Acid).
Trifluoroacetic Acid (TFA) is a synthetic organofluorine acid with the chemical formula C2HF3O2. It is a substitution derivative of acetic acid, with fluorine atoms replacing the hydrogen atoms of the acetyl group. It is also a much stronger acid than acetic acid. TFA is widely used in organic synthesis as well as ion pairing reagent or buffer in HPLC. It is corrosive and toxic to aquatic life and mammals, causing severe irritation and burns to skin, eyes, and the respiratory tract. Not only that, TFA is also highly mobile and persistent, leading to high retention of it in soil and water. Determination of it’s threat level on the environmental and health levels are still ongoing.
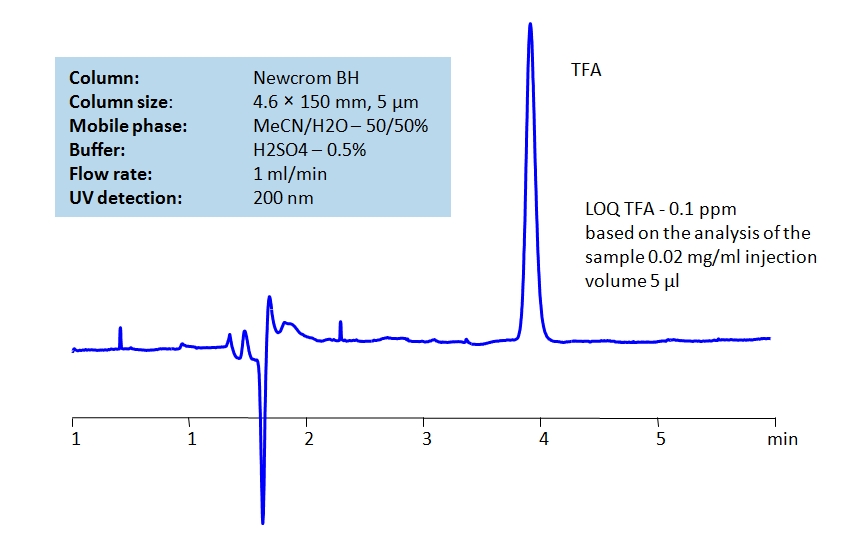
TFA can be detected at low UV. Using Newcrom BH mixed-mode column, a mobile phase of acetonitrile (ACN) and water with sulfuric acid (H2SO4) buffer can be used to retain TFA and UV detect it at 200nm.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | H2SO4 – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200nm |
| Class of Compounds | Acid |
| Analyzing Compounds | TFA (Trifluoroacetic Acid) |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended