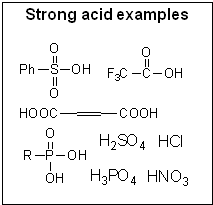
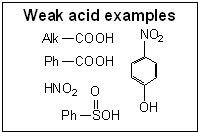
© SIELC Technologies. 2002 - 2025
Sign up to our newsletter
Contact
Address: 804 Seton Court, Wheeling, IL USA 60090
Tel: (847) 229-2629 | Fax: (847) 655-6079
Sales, Refund and Returns Policy
Email: mail@sielc.com | Sitemap
Host a Customized Seminar at Your Company
Delivering tailored seminars directly to your team.