| CAS Number | 110-86-1 |
|---|---|
| Molecular Formula | C5H5N |
| Molecular Weight | 79.102 |
| InChI Key | JUJWROOIHBZHMG-UHFFFAOYSA-N |
| LogP | 0.65 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Pyridine on Primesep 100 Column
August 12, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Pyridine on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Pyridine
Pyridine is an aromatic heterocyclic organic compound with the chemical formula C₅H₅N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom.
Pyridine can be retained, separated and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs a gradient method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV 250 nm.
You can find detailed UV spectra of Pyridine and information about its various lambda maxima by visiting the following link.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 70% |
| Buffer | H2SO4 – 0.05% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
| Limit of Detection | 5 ppb |
| Class of Compounds | Heterocyclic aromatic |
| Analyzing Compounds | Pyridine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

UV-Vis Spectrum of Pyridine
August 12, 2024
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Separation of Aminopyridines in Non-Aqueous Mobile Phase
July 10, 2013
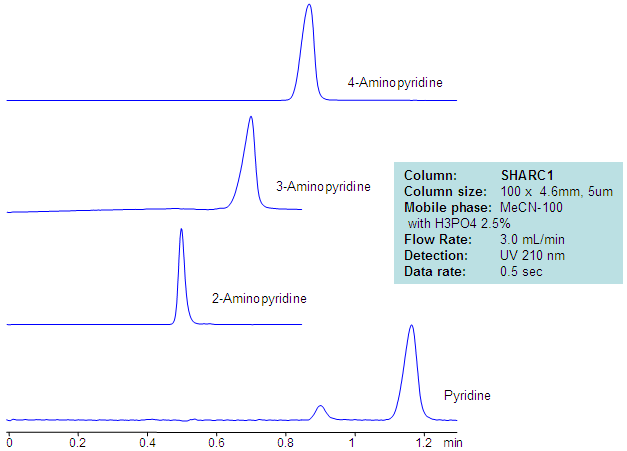
| Column | Sharc 1, 4.6×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 97.5% |
| Buffer | H3PO4 – 2.5% |
| Flow Rate | 3.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Muscle strengthener, Hydrophilic, Ionizable, Vertebrate pesticide |
| Analyzing Compounds | 2-Aminopyridine, 3-Aminopyridine, 4-Aminopyridine, Pyridine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select options3-Aminopyridine
4-Aminopyridine
Pyridine

HPLC Separation of Aminopyridines Isomers in Hydrogen-Bonding mode on a SHARC 1 HPLC Column
July 3, 2012
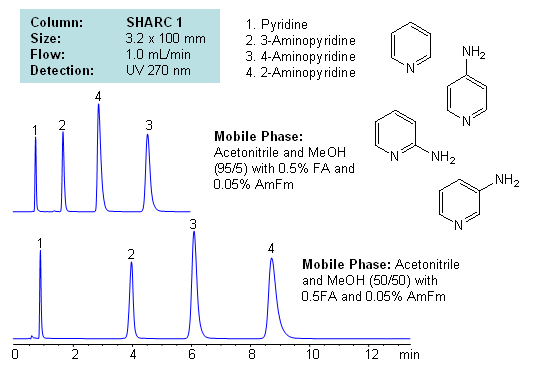
Application Notes: Pyridines and aminopyridines are hydrophilic basic compounds. Traditionally these compounds have been separated and analyzed by GC and HPLC. In the case of HPLC, reversed-phase chromatography with ion-pairing reagent is used along with alternative modes like HILIC for separation. Mixed-mode chromatography can also be used to successfully separate isomers of substituted pyridines. However, we developed a new mode of separation for these compounds with hydrogen bonding. Isomers of aminopyridine are separated based on hydrogen bonding interaction between analyte and stationary phase. Mobile phase utilizes combination of acetonitrile and methanol with additives. Retention time and selectivity are sensitive to variations of mobile phase. The order of elution changes depending on the amount of acetonitrile, methanol, formic acid and ammonium formate. This method and approach is compatible with LC/MS and prep chromatography and can be used for separation of other pyridine based compounds and pyridine based isomers.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A, To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: pyridine, 2-aminopyridine, 3-aminopyridine, 4-aminopyridine
Detection Technique: UV, LC/MS
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | Fa, AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Muscle strengthener, Hydrophilic, Ionizable, Vertebrate pesticide |
| Analyzing Compounds | 2-Aminopyridine, 3-Aminopyridine, 4-Aminopyridine, Pyridine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select options3-Aminopyridine
4-Aminopyridine
Pyridine
UV Detection

HPLC Separation of Amino Acids, Bases, Acids, and Neutrals on Obelisc R
March 3, 2007
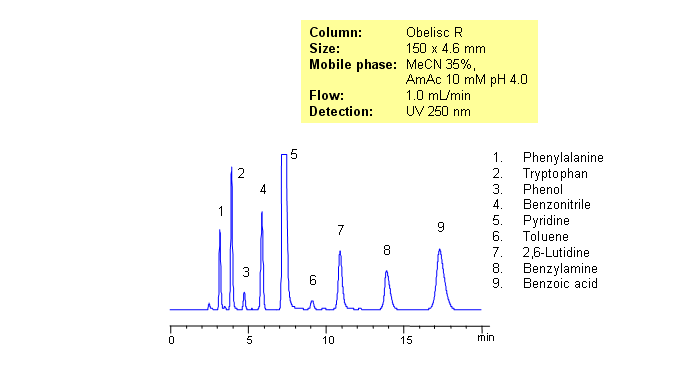
Separating basic, acidic and zwitterionic compounds in one run in reverse-phase HPLC can be very challenging. The methods might require the use of ion-pairing reagents and complex gradients that can make MS-compatibility difficult. Obelisc R column which has both positive and negative ion-pairs embedded in the stationary phase allows for fine tuning and separation of a wide range of compounds with different ionic properties. Acids, bases, amino acids and neutral compounds were separated isocratically in one run using a simple MS-compatible mobile phase of acetonitrile (ACN) and water with Ammonium Acetate (AmAc) buffer. Can also be UV detected at 250nm.
| Column | Obelisc R, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | AmAc 10 mM pH 4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Bases, Neutral, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Amino acids |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsBenzoic Acid
Benzonitrile
Benzylamine
Phenol
Phenylalanine
Pyridine
Toluene
Tryptophan

Complex Mixture of Acids, Bases, Amino Acids, and Neutral Compounds
October 14, 2006
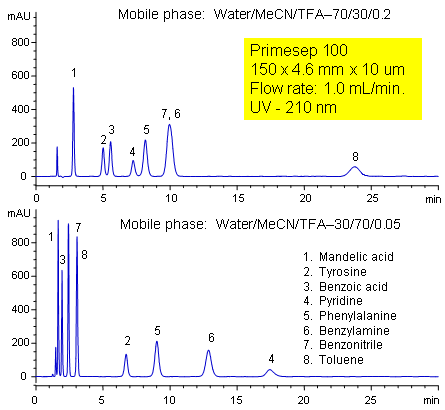
Primesep 100 separates a mixture of amino acids (tyrosine, phenylalanine), organic acids (benzoic acid, mandelic acid), amines (benzylamine, pyridine), and neutrals (benzonitrile, toluene) in one HPLC run by combining reversed-phase, cation-exchange, and polar interactions. The method is tunable and peak order can be changed significantly by adjusting acetonitrile and trifluoroacetic acid concentrations. The separation method uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) and compatible with UV, mass spec (LC/MS) and evaporative light scattering (ELSD) detection.
| Column | Primesep 100, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | TFA – 0.2 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Tyrosine, phenylalanine, Benzoic acid, mandelic acid, Benzylamine, Pyridine, Benzonitrile, Toluene |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzoic Acid
Benzonitrile
Benzylamine
Mandelic Acid
Organic Acids
Phenylalanine
Pyridine
Toluene
Tyrosine